|
© Borgis - Postępy Nauk Medycznych 11, s. 769-774
*Agnieszka Baranowska-Bik1, Agata Popielarz-Grygalewicz2, Marek Dąbrowski2, 3, Wojciech Zgliczyński1
Ocena echokardiograficzna parametrów lewej komory u pacjentów z zespołem Cushinga
Echocardiographic evaluation of left ventricular parameters in patients with Cushing’s syndrome
1Department of Endocrinology, Medical Center of Postgraduate Education, Bielański Hospital, Warszawa
Head of Department: prof. Wojciech Zgliczyński, MD, PhD 2Department of Cardiology, Bielański Hospital, Warszawa Head of Department: prof. Marek Dąbrowski, MD, PhD 3Department of Cardiology, Faculty of Physiotherapy, Warsaw Medical University Head of Department: prof. Marek Dąbrowski, MD, PhD Streszczenie
Wstęp. Zespół Cushinga charakteryzuje się szerokim spektrum zaburzeń metabolicznych i powikłań ogólnoustrojowych. Pacjenci z zespołem Cushinga, niezależnie od przyczyn hiperkortyzolemii, mają zwiększone ryzyko wystąpienia chorób sercowo-naczyniowych oraz wyższą śmiertelność. Wykazano związek pomiędzy hiperkortyzolemią a różnorodnymi zmianami funkcjonalnymi i strukturalnymi mięśnia serca stwierdzanymi w badaniu echokardiograficznym. Cel pracy. Celem pracy była ocena retrospektywna stanu metabolicznego i zmian echokardiograficznych lewej komory serca u pacjentów z zespołem Cushinga. Materiał i metody. Badaniu poddano 10 chorych z zespołem Cushinga (8 kobiet i 2 mężczyzn) w wieku 22-70 lat (śr. 45,8 lat ± 14,2). W 8 przypadkach hiperkortyzolemia była spowodowana ACTH-zależnym zespołem Cushinga (w tym 6 osób miało gruczolaka przysadki, a 2 ektopowe wydzielania ACTH), a u pozostałych 2 pacjentów – ACTH-niezależnym zespołem Cushinga (gruczolaki nadnercza). Wszyscy badani byli w aktywnej fazie choroby. Oceniano częstość występowania nadwagi/otyłości, nadciśnienia tętniczego, cukrzycy oraz dyslipidemii. Przeprowadzono analizę parametrów lewej komory uzyskanych w badaniu echokardiograficznym. Wyniki. W badanej grupie stwierdzono wysoką częstość występowania nadwagi/otyłości, cukrzycy, dyslipidemii oraz nadciśnienia tętniczego. Frakcja wyrzutowa i wymiary lewej komory były w granicach normy w całej grupie. U prawie wszystkich chorych stwierdzono nieprawidłowości parametrów lewej komory, m.in. zwiększony wymiar przegrody, wzrost grubości tylnej ściany oraz nieprawidłową masę z podwyższonym wskaźnikiem masy lewej komory. Wnioski. Zaburzenia czynności i zmiany strukturalne lewej komory są często obserwowane w zespole Cushinga, zatem badanie echokardiograficzne powinno być wykonywane u wszystkich pacjentów z hiperkortyzolemią. Słowa kluczowe: echokardiografia, struktura lewej komory, czynność lewej komory, zaburzenia metaboliczne, zespół Cushinga
Summary
Introduction. Cushing’s syndrome is characterized by the wide spectrum of metabolic abnormalities and systemic complications. Patients with Cushing’s syndrome, regardless of the cause of hipercortisolism, have enhanced cardiovascular risk and increased mortality rate. An association between hipercortisolism and a variety of cardiac functional and structural changes seen in echocardiography was previously reported. Aim. To retrospectively assess the metabolic status and echocardiographic alterations of left ventricle (LV) in patients with Cushing’s syndrome. Material and methods. The studied group consisted of 10 subjects (8 females and 2 males) with Cushing’s syndrome, aged 22-70 yrs (mean 45.8 yrs. ± 14.2). In 8 cases hipercortisolism resulted from ACTH-dependent Cushing’s syndrome (6 cases of pituitary adenoma and 2 cases of ACTH ectopic secretion) and other 2 were caused by ACTH-independent Cushing’s syndrome due to adrenal lesion. All of the subjects were in the active phase of disease. The prevalence of overweight/obesity, hypertension, diabetes and dyslipidemia was assessed. Analysis of left ventricular parameters obtained in echocardiography was performed. Results. Our studied group was found to have high prevalence of overweight/obesity, diabetes, dyslipidemia and hypertension. Ejection fraction and left ventricular dimensions were within normal range in entire group under the study. Almost all study participants presented abnormalities in left ventricular parameters including enhanced septum diameter, increased posterior wall thickness and relative wall thickness as well as LV mass and LV mass index out of normal range. Conclusions. As the left ventricular dysfunction and structural changes are commonly found in Cushing’s syndrome, echocardiographic evaluation should be performed in all patients with cortisol overproduction. Key words: echocardiography, LV structure, LV function, metabolic impairment, Cushing’s syndrome
Introduction
Cushing’s syndrome is characterized by the wide spectrum of metabolic abnormalities and systemic complications. Enhanced secretion of cortisol is associated with higher prevalence of dyslipidemia, hypertension, obesity and diabetes. The existence of those abnormalities is responsible for impaired metabolism, namely metabolic syndrome. It is widely known that metabolic disturbances are risk factors of cardiovascular events. It should be highlighted that patients with Cushing’s syndrome, regardless of the cause of hipercortisolism, have enhanced cardiovascular risk as a result of unfavorable effect of increased levels of circulating cortisol especially exerted on the heart and vasculature. In addition, cortisol induces chronic prothrombotic state. These features contribute to increase in mortality rate, estimated as even fourfold, observed among the patients with Cushing’s syndrome. Untreated or improperly treated Cushing’s syndrome is correlated with premature death. The main causes of death in this group of patients are as follows: cardiovascular disease including coronary heart disease, cardiac failure and thromboembolic complications (1-3). Several previous studies have presented echocardiographic evaluation of cardiac structure and function in patients with Cushing’s syndrome. An association between hipercortisolism and a variety of cardiac abnormalities including left ventricle hypertrophy, increased relative wall thickness and diastolic dysfunction have been found (4, 5).
Aim
Therefore, we aimed to retrospectively assess the metabolic status and echocardiographic alterations of left ventricle (LV) in patients with Cushing’s syndrome of various origin.
Material and methods
Study design
The study pattern was set as retrospective analysis.
Study population
Ten subjects with diagnosed endogenous hipercortisolism were randomly selected amongst patients with Cushing’s syndrome that were referred to the Department of Endocrinology of Bielański Hospital, Warsaw, Poland in a period of 12 months (years: 2012/2013).
The studied group consisted of 10 patients (8 females and 2 males) aged 22-70 years old. The mean age was 45.8 yrs ± 14.2. The diagnose of Cushing’s syndrome was made in accordance with standard criteria. There were 8 subjects with ACTH-dependent Cushing’s syndrome (6 cases of pituitary adenoma and 2 individuals with ACTH ectopic secretion) while 2 other cases were ACTH-independent Cushing’s syndrome in a course of adrenal lesion. All of the subjects were in the active phase of disease during hospitalization and all of them had a history of hipercortisolism treatment failure due to unsuccessful surgery or ineffective pharmacological treatment (with ketoconazole as a blocker of adrenal steroidogenesis).
The detailed study population characteristic is presented in table 1.
Table 1. Study population characteristic.
Based on the medical history and laboratory results the metabolic status and the prevalence of overweight/obesity, hypertension, diabetes and dyslipidemia were assessed. The results are presented in table 2 and table 3, respectively. The diagnoses of hypertension, diabetes and dyslipidemia were based on current criteria. All individuals signed informed consent for hospitalization and medical procedures.
Table 2. Anthropometric and biochemical parameters, with list of concomitant medications.
N/a – not applicable; ARB – angiotensin receptor blocker; ACEi – angiotensin converting enzyme inhibitor
Table 3. The prevalence of overweight/obesity, hypertension, diabetes and dyslipidemia.
Anthropometric measurement
The anthropometric measurements, including weight and height, were performed on the first day of hospitalization. The body mass index (BMI) was calculated according to the formula: body mass [kg]/weight [m] x weight [m]. In accordance with BMI results study participants were stratified as normal weight [BMI < 25 kg/m2], overweight [BMI between 25 and 29.9 kg/m2] or obese [BMI > 30 kg/m2] individuals.
Analytical methods
The blood samples were collected after at least 6 hours of fasting. Glucose and lipid parameters were measured in sera with use of routine laboratory tests.
Echocardiography
The echocardiographic evaluation was carried out in the Department of Cardiology of Bielański Hospital. Echocardiography was performed in all individuals in a transthoracic manner with use of 3.5 MHz transducer (Vivid 4 and Vivid 9).The following parameters were analyzed among others: LV end-diastolic and end-systolic diameter, intraventricular septum and posterior wall thickness as well as EF (ejection fraction). Moreover, LV mass index was calculated in reference to body surface area and LV mass was estimated. In addition, RWT (end-diastolic relative wall thickness) was also assessed to indicate an index of LV concentric geometry. The evaluation of diastolic function was performed as transmitral flow E-wave velocity (E) and A-wave velocity (A) was assessed using pulse-wave Doppler method, and then the E/A ratio was calculated. Early diastolic peak velocity (E’) was also measured at the septal level of mitral valve annulus and then E/E’ ratio was estimated, reflecting LV filling pressures. The detailed outcomes of echocardiographic examination are shown in table 4.
Table 4. Echocardiographic assessment of left ventricular structure and function.
EF – ejection fraction; LVEDd – left ventricular end-diastolic diameter; LVESd – left ventricular end-systolic diameter; IVSd – intraventricular septum diastolic diameter; PWd – posterior wall diastolic diameter; RWT – relative wall thickness; E – transmitral flow E-wave velocity; A – transmitral flow A-wave velocity; E’ – early diastolic peak velocity
Data analysis
Continuous variables are presented as mean ± SD.
Results
The basic characteristic of the studied group revealed that 5 out of 10 subjects were exposed to increased levels of cortisol for less than 5 years, in one case exposition time was between 5 and 10 years and in 5 other cases hipercortisolism lasted at least 10 years.
Enhanced body weight was found in 8/10 investigated individuals and, additionally, mean BMI of entire group was 31.98 ± 8.5 kg/m2.
Diabetes was seen in 8 individuals. Analysis of anti-diabetic treatment revealed that diabetes was treated with metformin in 4 cases, and with insulin in 3 cases, but mixed therapy with insulin and metformin was performed in one case. One patient received sulfonyl urea treatment and one was on diet only.
Hypertension was present in 9 subjects. All of them were receiving hypotensive treatment. In details, 5 individuals needed monotherapy, but other 4 were treated with at least 3 antihypertensive medications.
Abnormalities in lipid profile or prior lipid-lowering treatment were noticed in 8 out 10 study participants.
The echocardiography examination revealed that ejection fraction and left ventricular dimensions were within normal range in entire group under the study. Only in one case inappropriate E/E’ ratio was observed. Almost all study participants (9/10) had enhanced septum diameter, and 80% had increased posterior wall thickness. The examples of echocardiographic findings are presented in figure 1 and figure 2. In 9 patients RWT was seen higher than normal. Abnormalities in LV mass as well as in LV mass index were noticed in 8 individuals confirming left ventricular hypertrophy.
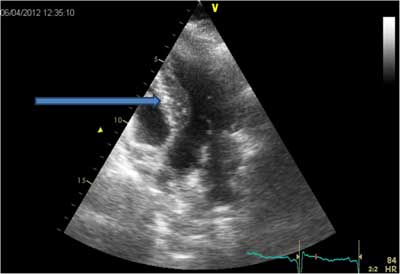 Fig. 1. Increased intraventricular septum diastolic diameter in patient TC.
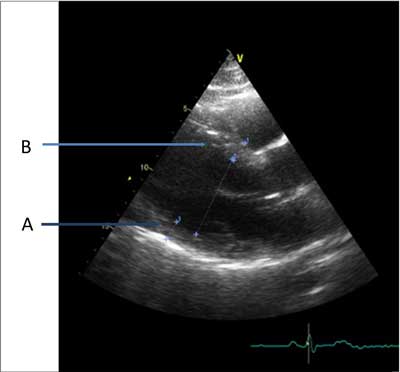 Fig. 2. Increased posterior wall thickness (A) and intraventricular septum diastolic diameter (B) in patient JG.
Analyses were performed in accordance to references (5-7).
Interestingly, we observed that normal echocardiography outcome was found in individual with a long hipercortisolism history.
Discussion
The results of our study revealed that most of the patients with Cushing’s syndrome do not only suffer from the metabolic and systemic complications but also a have left ventricle abnormalities found in echocardiographic examination. Although data concerning individuals with Cushing’s syndrome had been previously published, our study material comprised of subjects in whom hipercortisolism was present for several months or even years as surgical and/or pharmacological therapy with ketoconazole as a blocker of adrenal steroidogenesis failed to be completely sufficient.
Our studied group was found to have high prevalence of components of metabolic syndrome: inappropriate body mass index, diabetes, dyslipidemia and hypertension.
Data from literature indicate that 2/3 patients with Cushing’s syndrome present metabolic abnormalities with at least 3 features of metabolic syndrome. In accordance to previous observations glucose homeostasis dysfunction is frequently found in subjects with cortisol overproduction as 20-60% patients have impaired glucose tolerance and 20-47% individuals are diagnosed with diabetes (8). In our material diabetes was found to be more common as 8 from 10 patients had diagnosis of diabetes. The key mechanism influencing carbohydrate metabolism in this disease is insulin resistance that also impact on cardiovascular system. It has been reported that insulin resistance leads to sodium and water retention (9). On the other hand, hiperinsulinemia impacts sympathoadrenal system activity, induces local renin-angiotensin-aldosterone system activation and vascular hypertrophy. Consequently, hiperinsulinemia and insulin resistance contribute to vascular resistance and hypertension (9).
Obesity with typical fat redistribution is also a distinguishing feature of endogenous Cushing’s syndrome. We found that 8 of 10 our subjects were overweight or obese.
Anthropometric studies performed by other authors revealed that individuals with Cushing’s syndrome have increased waist to hip ratio (WHR), the marker that is recognized as a risk factor of cardiovascular diseases. Indeed, data from literature indicated that WHR correlates with blood pressure, basic and OGTT glucose levels and insulin concentration (10). Fat tissue, especially of visceral origin, has biological activity as it is able to produce and secrete several molecules, named adipokines. Adipokines may influence carbohydrate and lipid metabolism, and indirectly affect cardiovascular system (11).
Hypertension was observed in 9 patients (90%) from the studied group. It has been reported previously that in endogenous Cushing’ s syndrome the prevalence of hypertension is about 80% in adults and approximately 47% in children (9). The mechanism of increased blood pressure in hipercortisolism is complex.
Cicala and Mantero presented detailed mechanisms of hypertension accompanying cortisol excess. Briefly, impairment of mechanisms regulating plasma volume, peripheral vascular resistance and cardiac output play a role in pathomechanism of increased blood pressure. Moreover, enhanced levels of glucocorticoids may exert hypertensive effect in other mechanism including mineralocorticoid activity, activation of the renin-angiotensin system, enhancement of vascular reactivity to vasoconstrictors, increased beta-adrenergic receptor sensitivity to catecholamines and suppression of the vasodilatory system (9).
When analyzing results of echocardiographic examinations, we observed that amongst our patients the most common abnormalities were as follow: enhanced septum diameter, increased posterior wall thickness, abnormal relative wall thickness and LV mass as well as LV mass index out of the normal range. Data from literature concerning echocardiographic outcome in patients with hipercortisolism are ambiguous.
Toja et al. presented results of echocardiographic evaluation of patient’s with Cushing’s syndrome before and after cure. In comparison with controls individuals those patients with cortisol excess presented abnormal left ventricle mass parameters with enhanced LV mass index seen in 46% of cases (vs. 19% in the controls). Intraventricular septum thickness measurement revealed that this parameter was increased in 69% of patients with Cushing’s syndrome (vs. 39% in the controls). Similarly, posterior wall thickness was found to be increased in almost half of the patients with cortisol overproduction (vs. 9% of the controls). Finally, RWT was significantly higher in case of hipercortisolism. Ejection fraction was comprised within normal range in almost all cases. Also E/A ratio as a marker of diastolic function remained unchanged. Those authors also assessed the influence of remission of hipercortisolism on cardiac structure and function abnormalities seen in echocardiography. Left ventricular mass parameters ameliorated considerable; however, they still were increased as compared with the controls (2).
The group of Fallo et al. reported that patients with Cushing’s syndrome in comparison with healthy controls presented significantly increased posterior wall thickness and relative wall thickness, while LV end-systolic diameter was increased. Those authors failed to find any significant changes amongst other echocardiographic measurements between patients suffering from cortisol excess and the controls (12).
Yiu et al. compared echocardiographic outcome of individuals with Cushing’s disease, primary hypertension and the controls. Data from this study showed that end-diastolic septal thickness and LV mass index did not differ between patients with hipercortisolism and those with hypertension, although these parameters were larger as compared with the controls. Results of LV dimensions and ejection fraction were comparable in all three groups. E/A and E/E’ ratios were significantly impaired in subjects with Cushing’s disease as well as in hypertensive individuals (13).
The results of research conducted by Pereira et al. showed that there were no differences in LV diameters, volumes, and ejection fraction between patients with Cushing’s syndrome and the controls. LV hypertrophy was noticeable in subjects with cortisol excess as significantly higher values of intraventricular septum and posterior wall thickness, LV mass index, and relative wall thickness were observed. Moreover, this particular group had impaired early LV relaxation, with significantly lower values of transmitral E-wave velocity and E/A ratio and significantly longer isovolumetric relaxation time. In addition, a significantly higher E/E’ ratio was also observed (14).
Data from our study and the results of other authors suggest that there is a strong correlation between cardiac structures abnormalities seen in echocardiography and Cushing’s syndrome. Unexpectedly one patient with a long history of the Cushing syndrome (>10 yrs) had normal outcome of echocardiography. Moreover, the significantly impaired diastolic function was not observed in the majority of the patients despite the fact that 9 out of 10 patients had hyperthrophy. Further studies are needed to understand all aspects of these findings.
We are aware of several limitations of the presented study including small size of the group, absence of the controls and the nature of the study (a retrospective analysis). Therefore, we plan to conduct a prospective research that would include larger group of patients suffering from Cushing’s syndrome.
Conclusions
Cushing’s syndrome is related with increased cardiovascular risk and enhanced mortality rate. As the left ventricular dysfunction and structural changes are commonly found, echocardiographic evaluation should be performed in all patients with cortisol overproduction. Piśmiennictwo
1. De Leo M, Pivonello R, Auriemma RS et al.: Cardiovascular disease in Cushing’s syndrome: heart versus vasculature. Neuroendocrinology 2010; 92 (suppl. 1): 50-54.
2. Toja PM, Branzi G, Ciambellotti F et al.: Clinical relevance of cardiac structure and function abnormalities in patients with Cushing’s syndrome before and after cure. Clin Endocrinol (Oxf) 2012; 76(3): 332-338.
3. Dekkers OM, Horvath-Puho E, Jorgensen JO et al.: Multisystem morbidity and mortality in Cushing’s syndrome: a cohort Study. J Clin Endocrinol Metab 2013; 98(6): 2277-2284.
4. Muiesan ML, Lupia M, Salvetti M et al.: Left ventricular structural and functional characteristics in Cushing’s syndrome. J Am Coll Cardiol 2003; 41: 2275-2279.
5. Nagueh SF, Appleton CP, Gillebert TC et al.: Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22(2): 107-133.
6. Lang RM, Bierig M, Devereux RB et al.: Recommendations for chamber quantification. Eur J Echocardiogr 2006; 7(2): 79-108.
7. Płońska-Gościniak E (red.): Standardy kardiologiczne 2013 okiem echokardiografisty. Wyd. I, Medical Tribune Polska, Warszawa 2013.
8. Chanson P, Salenave S: Metabolic syndrome in Cushing’s syndrome. Neuroendocrinology 2010; 92 (suppl. 1): 96-101.
9. Cicala MV, Mantero F: Hypertension in Cushing’s syndrome: from pathogenesis to treatment. Neuroendocrinology 2010; 92 (suppl. 1): 44-49.
10. Faggiano A, Pivonello R, Spiezia S et al.: Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab 2003; 88(6): 2527-2533.
11. Valassi E, Biller BM, Klibanski A, Misra M: Adipokines and cardiovascular risk in Cushing’s syndrome. Neuroendocrinology 2012; 95(3): 187-206.
12. Fallo F, Famoso G, Capizzi D et al.: Coronary microvascular function in patients with Cushing’s syndrome. Endocrine 2012; 43(1): 206-213.
13. Yiu KH, Marsan NA, Delgado V et al.: Increased myocardial fibrosis and left ventricular dysfunction in Cushing’s syndrome. Eur J Endocrinol 2012; 166(1): 27-34.
14. Pereira AM, Delgado V, Romijn JA et al.: Cardiac dysfunction is reversed upon successful treatment of Cushing’s syndrome. Eur J Endocrinol 2010; 162(2): 331-340.
otrzymano/received: 2013-09-17 zaakceptowano/accepted: 2013-10-30 Adres/address: *Agnieszka Baranowska-Bik Department of Endocrinology Medical Center of Postgraduate Education Bielański Hospital ul. Cegłowska 80, 01-809 Warszawa tel./fax: +48 (22) 834-31-31 e-mail: klinendo@cmkp.edu.pl Artykuł Ocena echokardiograficzna parametrów lewej komory u pacjentów z zespołem Cushinga w Czytelni Medycznej Borgis. |
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








