|
© Borgis - Postępy Nauk Medycznych 11, s. 762-768
*Izabella Czajka-Oraniec, Maria Stelmachowska-Banaś, Wojciech Zgliczyński
Obrazowanie okolicy przysadkowo-podwzgórzowej przy użyciu rezonansu magnetycznego u pacjentów z moczówką prostą pochodzenia centralnego
Magnetic Resonance Imaging of the Pituitary-Hypothalamic Region in the Patients with Central Diabetes Insidpidus
Department of Endocrinology, Medical Center of Postgraduate Education, Bielański Hospital, Warszawa
Head of Department: prof. Wojciech Zgliczyński, MD, PhD Streszczenie
Wstęp. Moczówka prosta (MP) pochodzenia centralnego jest chorobą charakteryzującą się poliurią i polidypsją, do których prowadzi nieprawidłowa synteza lub wydzielanie wazopresyny na skutek różnych stanów chorobowych uszkadzających podwzgórze, tylny płat przysadki lub szypułę. Postawienie prawidłowej diagnozy i określenie etiologii MP bywa trudne, zatem pomocne może być posłużenie się badaniem rezonansu magnetycznego (MR). Cel pracy. Celem pracy była ocena częstości występowania i etiologii moczowki prostej wśród pacjentów hospitalizowanych w Klinice Endokrynologii CMKP w Szpitalu Bielańskim w ostatnim roku oraz analiza wynikow wykonanych u nich badań MR okolicy podwzgórzowo-przysadkowej. Materiał i metody. Retrospektywne badanie obejmowało analizę dokumentacji medycznej. Wyszukano pacjentów hospitalizowanych w Klinice Endokrynologii CMKP w Szpitalu Bielańskim w ostatnim roku. Wyszukano pacjentów z rozpoznaniem moczówki prostej i zebrano dane dotyczące wieku, płci, przyczyn choroby i czasu jej trwania, wyników badań biochemicznych i hormonalnych oraz stosowanego leczenia. Analizowano również wyniki MR okolicy przysadkowo-podwzgórzowej. Wyniki. U 37 spośród 1724 (2,15%) pacjentów hospitalizowanych w Klinice rozpoznano MP, natomiast tylko 9 pacjentów miało świeże objawy poliurii i polidypsji wymagające ustalenia rozpoznania (0,5%). U większości pacjentów (67,5%) stwierdzano towarzyszącą niedoczynność przedniego płata przysadki. Najczęstszą przyczyną MR była przebyta operacja guza śród- lub okołosiodłowego. Idiopatyczną MP rozpoznano tylko w 2 przypadkach. Najczęstszą zmianą stwierdzaną w MR był brak świecenia tylnego płata przysadki. Poza tym często spotykanymi nieprawidłowościami były: pogrubienie szypuły, guzy i puste siodło. Wnioski. Moczówka prosta była rzadkim rozpoznaniem u pacjentów Kliniki. W większości przypadków szczegółowe wywiady, badanie fizykalne i badanie MR okolicy przysadkowo-podwzgórzowej pozwalały na określenie etiologii MP. Obserwowaliśmy mniej przypadków MP idiopatycznej niż opisywano w literaturze, a większość pacjentów miało MR wtórną do leczenia neurochirurgicznego dużych śród- i okołosiodłowych guzów. Słowa kluczowe: przysadka, tylny płat (nerwowy) przysadki, wazopresyna, moczówka prosta, rezonans magnetyczny
Summary
Introduction. Central diabetes insipidus (DI) is a disease characterized by polyuria and polydipsia resulting from various pathologic processes and lesions destroying hypothalamus pituitary stalk or neurohypophysis that impair vasopressin synthesis or secretion. Making the proper diagnosis and finding the DI etiology is sometimes challenging and magnetic resonance (MR) evaluation could be a helpful tool. Aim. We intended to investigate the prevalence and causes of DI and to analyse the MRI results in patients hospitalized at the Department of Endocrinology in Bielański Hospital during last year. Material and methods. We performed a retrospective review of the medical data of patients hospitalized in the Department of Endocrinology in Bielański Hospital during last year searching for those with the diagnosis of DI. We collected data on the age, sex, causes of DI, disease duration, results of hormonal and biochemic tests and applied treatment and we evaluated the results of pituitary-hypothalamic region. Results. Among 1724 patients hospitalized in our Department, 37 (2.15%) had DI and only 9 patients had new-onset polyuria and polydipsia (0.5%). Majority of patients had concurrent hypopituitarism (67.5%) and the most frequent cause of DI was prior surgical treatment for large sellar or parasellar tumours. Idiopatic DI was found only in 2 cases. The most consistent finding on MR images was the loss of posterior pituitary bright spot. Other abnormalities such as pituitary stalk thickening, tumours and empty sella were commonly detected. Conclusions. DI was a rare clinical condition in our patients. In most cases detailed medical history, physical examination and MR of pituitary-hypothalamic region allowed to find its etiology. We observed less cases of idiopathic DI than it was described in the literature and majority of studied group had DI secondary to prior neurosurgical treatment for large sellar and parasellar tumours. Key words: pituitary, neurohypophysis, vasopressin, diabetes insipidus, magnetic resonance imaging
Introduction
Central diabetes insipidus (DI) is an inherited or most commonly acquired multifactorial disease characterized by the excertion of the large volume of diluted, hypotonic urine and as a consequence excessive thirst and fluid intake (polyuria and polydipsia) (1). Vasopressin insufficiency is the reason of above symptoms. It results from inadequate synthesis or secretion of arginine vasopressin (AVP) or antidiuretic hormone (ADH) which is a peptide hormone synthesized by neuronal bodies that form supraoptic and paraventricular nuclei of the hypothalamus. Once synthesized and packed in neurosecretory granules, AVP is transported along axons through the stalk to the posterior pituitary (neurohypophysis), where it is stored for further secretion. Various pathologic processes and lesions that destroy the sites of vasopressin synthesis, transport or storage (hypothalamus, pituitary stalk or neurohypophysis) could lead to the symptoms of diabetes insipidus. The known causes include sellar and suprasellar tumours (the most commonly craniopharyngioma), granulomatous diseases such as Langerhans cell histiocytosis and sarcoidosis, trauma from neurosurgery or accidents, inflammatory and autoimmune diseases such as lymphocytic infundibulo-hypophysitis. In up to 50% of patients, particularly children and young adults the etiology of DI remains unknown and in such cases DI is termed idiopathic (2).
Magnetic resonance imaging (MRI) is the best and the most precise method for imaging the pituitary gland and hypothalamus and the endocrine diseases originating in that region (3). MR images of the pituitary gland show not only detailed anatomy of anterior and posterior pituitary lobes (adenohypophysis and neurohypophysis), pituitary stalk and infundibulum but they allow to conclude on the posterior pituitary function as well. The normal content of vasopressin packed in neurosecretory granules and stored in neurohypophysis is responsible for the high signal intensity of the posterior pituitary gland in pre-contrast T1-weighted MR images (4). Therefore, in most cases of normal posterior pituitary function the hyperintense signal in the posterior part of the sella turcica is present (called posterior pituitary bright spot). On the other hand, in most cases of central diabetes insipidus the bright spot is absent (5).
Aim
The aim of the study was to retrospectively review the cases of the central diabetes insipidus among the patients hospitalized at the Department of Endocrinology of The Centre of Postgraduate Medical Education in Bielański Hospital during last year. We evaluated the prevalence and causes of the disease, with a detailed analysis of the magnetic resonance imaging of the pituitary-hypothalamic region results.
Material and methods
We retrospectively reviewed the electronic medical records of patients hospitalized at the Department of Endocrinology in Bielański Hospital during last year (between September 2012 and September 2013) searching for the patients with the ICD-10 codes: D35.2 (benign neoplasm of pituitary gland) and E23. That included patients with following codes: E23.0 (hypopituitarism), E23.1 (iatrogenic hypopituitarism), E23.2 (diabetes insipidus), E23.6 (other disorders of pituitary gland). We searched electronic and paper documentation of those patients (n = 300) to find the cases with the diagnosis of diabetes insipidus (DI). Then, in that selected group of patients (n = 37) we collected data on the age, sex, causes of DI, disease duration, results of hormonal and biochemic tests and applied treatment. The results of current magnetic resonance imaging of pituitary-hypothalamic region, available in 32 cases, were analysed. We investigated the presence or absence of posterior pituitary bright spot on pre-contrast T1-weighted sagittal MR images, we looked for sellar or parasellar tumours and estimated the pituitary height and pituitary stalk thickness.
Additionally we analysed the presence or absence of the posterior pituitary hyperintensity on the MRI scans performed in 38 consecutive patients admitted to the Department of Endocrinology with no symptoms of diabetes insipidus.
Results
Group characteristic
During last year (from September 2012 to September 2013) one thousand seven hundred twenty four (1724) patients with numerous endocrine disorders were hospitalized at the Department of Endocrinology in Bielański Hospital. Among them 37 patients had the diagnosis of diabetes insipidus, which was 2.15% of all hospitalized patients in the Department of Endocrinology. The mean age was 43.2 ± 17.1 years (range from 18 to 80 years). There were 25 (67.6%) females and 12 (32.4%) males. Only nine patients with DI (24.3% of DI patients and 0.5% of all patients) have been hospitalized because of the symptoms of DI that had appeared recently (less than half a year before hospitalization) and the new diagnosis of DI has been established or the suspicion has been confirmed in all but one patient. Diagnoses in most cases were based on symptoms (hypotonic polyuria and polydipsia > 4 liters/24 h) and decreased urine osmolality, that resolved following desmopressin administration, if diabetes mellitus, hypercalcemia, hypokaliemia and renal disease were excluded.
Majority of patients (75.7% of DI patients) had well controlled permanent diabetes insipidus that lasted for years (on average for 7 years, approximately) and the reason for hospitalization was to assess the progress of the underlying disease and to control efficiency of treatment. Duration of the disease varied from couple of weeks to 21 years; between half a year and one year in four patients, between one year and five years in six patients, between five and ten years in three patients, between ten and twenty years in ten patients and longer than twenty years in three patients. One patient had transient postoperative DI that resolved in 3 months, and a patient with gestational diabetes insipidus had a spontaneous remission after delivery.
Twenty five patients with DI (67.6%) had pituitary insufficiency. Among them majority of patients (n = 18) had panhypopituitarism; in 16 patients as a result of pituitary-hypothalamic tumours or past surgery, in one case with limfocytic hypophysitis and in one case with metastatic pituitary lesion. In 7 patients we observed partial deficiency of pituitary hormones: adrenocorticotropic hormone (ACTH) in 3 cases, thyrotropin (TSH) and gonadotropins (FSH and LH) in 2 cases and gonadotropins only in 2 cases. There were 11 patients with normal function of anterior lobe of the pituitary gland. In the group with normal adenohypophysis function there were patients with posttraumatic, idiopathic and gestational DI, patients with encephalitis and meningitis in anamneses, one case of histiocytosis and one patient after two operations of a large sellar tumour.
Seven patients had various visual disturbances following craniotomy for large suprasellar tumours: visual field defects, diplopia and even blindness.
Etiology of diabetes insipidus
The causes of DI in studied group are listed in table 1. In almost two-thirds of our patients, diabetes insipidus was the consequence of prior surgery of the pituitary-hypothalamic tumours or the presence of the tumour itself. The most frequent type of tumours in our group were pituitary adenomas (in 9 cases, 24.3%) but in all these cases the DI was a complication of the surgical treatment not the tumour itself. Similarly, diabetes insipidus appeared usually postoperatively in other patients with pituitary-hypothalamic tumours such as craniopharyngiomas, Rathke’s cleft cysts, meningioma and other not classified tumours, although we had not precise data on the time of DI appearance in each case. Diabetes insipidus was one of the first symptoms of the disease in one patient with Rathke’s cleft cyst and in another with pituitary metastatic lesion.
Table 1. Etiology of diabetes insipidus in 37 patients hospitalized in the Department of Endocrinology between September 2012 and September 2013 with recognized previously central diabetes insipidus (n = 28) or new onset of polydipsia/polyuria syndrome (n = 9).
Only one third of the studied group suffered from DI not related to the sellar or suprasellar tumours. We found two cases of Langerhans cell histiocytosis, two lymphocytic hypophysites, two posttraumatic and two post-inflammatory DI (meningitis and encephalitis in anamneses). In single cases we identified gestational DI and DI caused by primary empty sella syndrome (PESS). In two patients the cause of the disease remained unknown (diagnosed as idiopathic DI) and in one patient we excluded central DI and diagnosed primary psychogenic polydipsia.
Treatment and disease control
All patients but one with primary psychogenic polydipsia, have been treated with vasopressin analogue – desmopressin (DDAVP) and data on doses used were available for 33 patients. Twenty five patients have been treated with sublingual tablets (Minirin MELT) with the mean daily dose approximately 150 mcg (range from 30 to 480 mcg, 1 to 4 times per day). Eight patients have been taking desmopressin nasally (Minirin) in doses ranging from 10 to 40 mcg daily (mean 23.75 ug, once or twice a day).
In five patients sodium concentration imbalances were present. We observed mild hyponatremia in 3 cases – (Na+): 131-133 mEq/L, moderate in one case – (Na+) = 129 mEq/L, and severe in one case – (Na+) = 121 mEq/L. In two later cases hyponatremia was corrected by reducing desmopressin dose and improving the control of secondary adrenal insufficiency. In the hospitalized group mean sodium concentration (Na+) was 138.3 ± 4.2 mEq/L and mean potassium concentration (K+) was 4.2 ± 0.4 mEq/L.
Magnetic Resonance Imaging results
MRI of pituitary-hypothalamic region was available for evaluation in 32 cases. The posterior pituitary bright spot on T1-weighted images was absent in all but two cases: one patient with psychogenic polydipsia and one patient with transient DI following transsphenoidal surgery for a large inactive adenoma who was examined 3 months after operation when DI symptoms resolved. Another patient with posttraumatic DI had some residual hyperintesity of the posterior pituitary present on the MR images performed 3 days after traffic accident, although he had persistent DI during our evaluation a month afterward. We did not repeat the MR scan then.
To compare the results obtained in patients with diabetes insipidus we analysed the signal intensity of neurohypophyses in 38 consecutive patients admitted to the Department with no symptoms of DI, who had a pituitary MR performed for numerous reasons. The posterior pituitary bright spot was absent in 6 patients (16.2%), all of them had history of surgery for pituitary adenoma but no current symptoms or treatment for DI. In 32 patients we identified the posterior pituitary hyperintense signal, although it was weak or moderate in 11 patients, mostly those previously surgically treated for pituitary tumours. Twenty one patients had a distinct bright spot of the neurohypophysis – 8 cases with no anomaly in pituitary-hypothalamic region, 6 patients with pituitary adenomas present and 5 patients who had been operated for pituitary and suprasellar tumours.
In majority of patients who undergone surgery for large pituitary or suprasellar tumours we observed the empty or partially empty sella (n = 13), with the pituitary height diminished to less than 4 mm or even less then 2 mm (flattened gland lying often in a deformed and enlarged sella turcica). In those cases the posterior pituitary bright spot was absent as well. Primary empty sella was observed in a 60-years-old man, together with thickened pituitary stalk and anterior pituitary hormones deficiency. In that case the etiology of the disease was not explained.
Four patients after surgery for large tumours had residual tumours, among them two patients with giant and aggressively growing pituitary adenomas.
Pituitary stalk thickening (PST), defined as the stalk diameter of more than 3 mm (6), was found in patients with Langerhans cell histiocytosis (n = 2), lymphocytic hypophysitis (n = 2), primary empty sella syndrome (n = 1), metastatic tumour and one case of idiopathic diabetes insipidus.
In one female patient with panhypopituitarism and DI following surgery for craniopharyngioma the pituitary stalk was absent, probably it was damaged during operation.
The MRI of pituitary-hypothalamic region was completely normal in a patient with primary psychogenic polydipsia and normal but with no posterior pituitary hyperintensity in a pregnant woman, in two men after head trauma (one recent and another in the early childhood) and in one case of idiopathic DI.
Figures 1 to 8 demonstrate MR images of the pituitary-hypothalamic region in a healthy individual and in 7 patients selected from the studied group with central diabetes insipidus of various etiology.
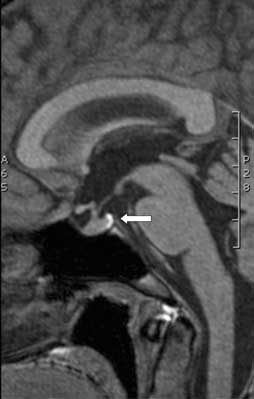 Fig. 1. Sagittal pre-contrast T1-weighted MR image showing normal pituitary gland with posterior pituitary bright spot pointed by arrow.
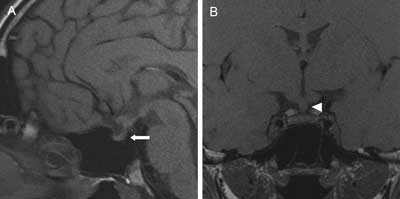 Fig. 2A, B. T1-weighted MR images showing a small pituitary gland with absent bright spot of the neurohypophysis (arrow) and thickened pituitary stalk (5 x 4.5 mm) (arrowhead) in 18-years-old man with sudden onset of polyuria and polydipsia (up to 10 liters per day) and general malaise and body weight loss of 4 kg two years ago. Before hospitalization in our hospital he was examined couple of times in pediatric wards and the final diagnosis of psychogenic polydipsia has been set because of self-willed interruptions of water deprivation tests and school-related problems. Patient was left without trial of desmopressin treatment with the excessive nycturia and polyuria persisting. After we introduced desmopressin he felt significant improvement, diuresis decreased to 2 liters a day and nycturia stopped. There was no deficiency in anterior pituitary function.
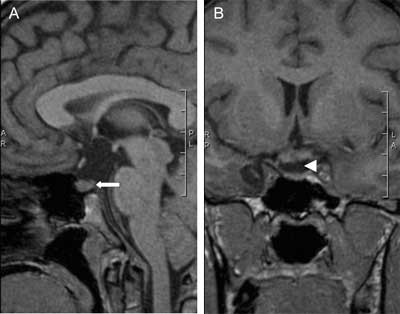 Fig. 3A, B. Sagittal and coronal pre-contrast MR images showing a pituitary gland with the loss of posterior pituitary bright spot (arrow) and absent pituitary stalk (arrowhead). Pituitary stalk was damaged during surgery for a large craniopharyngioma in 30-year-old woman and postoperatively diabetes insipidus together with panhypopituitarism appeared.
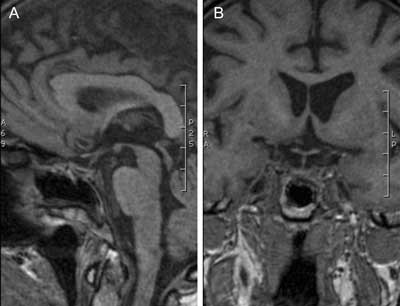 Fig. 4A, B. MR images showing the enlarged and partially empty sella turcica (pituitary height less than 4 mm) and absent neurohypophysis bright spot in 67-year-old woman after transsphenoidal surgery for macroadenoma secreting growth hormone 14 years ago. Diabetes insipidus and panhypopituitarism was observed since surgery but the patient is permanently cured from acromegaly.
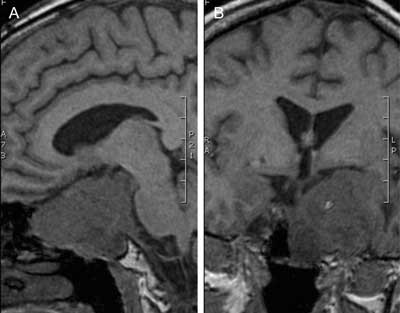 Fig. 5A, B. Pre-contrast MR images of the giant, invasive and agressively growing pituitary adenoma (gonadotropinoma) that had been neurosurgically treated two times in the past, leading to panhypopituitarism and diabetes insipidus in a 52-years-old woman.
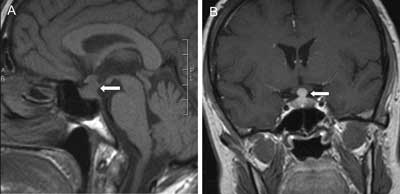 Fig. 6A, B. Sagittal T1-weighted pre-contrast and coronal contrast-enhanced MR images showing pituitary stalk thickening and pituitary enlargement (arrows) in 59-year-old woman with lymphocytic infundibulo-neurohypophysitis leading to multiple pituitary hormone deficiency and diabetes insipidus. Lack of the posterior pituitary bright spot in sagittal section is consistent with the diagnosis of central DI. The lymphocytic infiltration of the pituitary gland regressed following 10 months treatment with prednisone but thickening of the stalk persisted together with the pituitary dysfunction. The treatment was stopped because of poor clinical outcome and drug side effects.
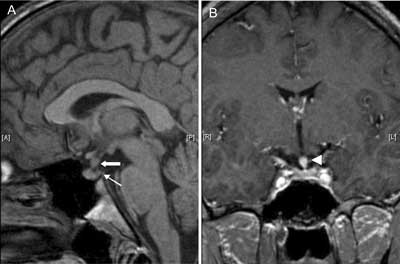 Fig. 7A, B. MR images demonstrating a granulomatous mass causing thickening of pituitary stalk (arrow) that markedly enhances after gadolinium (7B, arrowhead) and absent posterior pituitary bright spot (thin arrow) in 20-year-old man with DI that was a main endocrine symptom of systemic Langerhans cell histiocytosis. Diagnosis of the disease was set by dermatologists from histopathologic examination of the skin lesions. Apart from vasopressin deficiency patient had isolated gonadotropin deficiency leading to hypogonadism that was corrected with testosterone therapy.
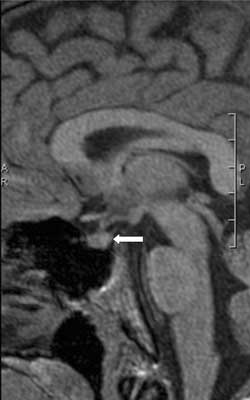 Fig. 8. Sagittal T1-weighted MR image demonstrating normal anterior pituitary and stalk and absent posterior pituitary bright spot in 19-years-old man with 17 years history of diabetes insipidus following head trauma in early childhood. Anterior pituitary function has always been normal and the patient had well controlled DI with sublingual desmopressin.
Discussion
Our data confirm that central diabetes insipidus is a rare endocrine disorder. There are scant data on the incidence and prevalence of DI but approximately 1 in 25 000 in general population is affected (1). Among patients hospitalized during one year in our Department of Endocrinology which is one of the leading endocrine centres in the country located in the trietary medical centre, only 2.15% of patients had diabetes insipidus and the majority of them (75.7%) had the disease recognised long before the current admission. There were only 9 cases (0.5%) of new onset polyuria-polydipsia syndrome requiring further diagnosis to confirm or exclude central DI and to establish treatment. One of the reasons, apart from rarity of the condition, could be the fact that the most frequent age of onset is between 10 and 20 years, so such patients with DI are mainly diagnosed by pediatricians (7).
Patients with permanent DI that had already lasted for years were usually well controlled with desmopressin. Only few patients (n = 5) had hyponatremia that was easily correctable by lowering desmopressin dosage. No other side effects were noted, proving the treatment to be safe and well tolerated. Mean doses of sublingual and intranasal desmopressin taken by our patients were in the lower ranges. On one hand it could be the result of partial AVP deficiency in many patients, on the other hand lower doses make the treatment safer, as in general it is thought that undertreatment with desmopressin is safer than overtreatment (8).
We found that the etiology of diabetes insidipdus in the studied group is to some degree different that usually described, especially in pediatric population (9). Patients with postoperative DI were overrepresented and cases of idiopathic DI were underrepresented in our group, although if we consider only new cases of DI, there were 2 patients with idiopathic DI out of 9 (22.2%). That was close to the numbers reported by some authors (6). Higher incidence of idiopathic DI was described in pediatric populations (2, 10). In the group described in that paper, careful history taking and physical examination, together with the MR images assessment helped in establishing the final diagnosis in most cases. Such evaluation and demonstration of the excretion of large volume of diluted urine (more than 4 litres per day of urine with a low specific gravity and osmolality) with a significant reduction of urine volume and increase in its specific gravity and osmolality following desmopressin administration were sufficient to establish DI diagnosis in most patients. Thus we could omit a dehydration test that is difficult and demanding for the patients and the medical stuff.
We observed a high frequency of anterior pituitary insufficiency in the studied group (67.6%). It was primarily associated with prior neurosurgical treatment, especially for the large tumours such as cranipharyngiomas or Rathke’s cleft cysts (11, 12). Other identified causes of hypopituitarism were: histiocytosis, lymphocytic hypophysitis, metastatic lesion and primary empty sella. That is in accordance with the data from literature (13-16). However we did not find the association between posttraumatic DI and hypopituitarism but there were only two patients in our observation. Krahulik et al. (17) recommend routine pituitary hormone testing and follow-up in such cases because the incidence of hypopituitarism is high and the onset could be delayed. We found that our two patients with idiopathic DI had normal anterior pituitary function, which correspond well with Hannon et al. data (18) but the number of patients was too low to draw a conclusion.
Magnetic resonance imaging is the crucial examination in patient with the suspicion of central diabetes insipidus (1, 3, 5, 6). We found that in all but one patient with clinically confirmed active central diabetes insipidus the hyperintense signal of posterior pituitary was lost. The patient with preserved, residual signal had the MR scan performed at the very early stage of DI (on 3rd day after head trauma) and the timing could be the reason of such MR image (6). Thus the absence of posterior pituitary bright spot could strongly suggest the diagnosis of DI. However, we showed as well that it could be lost in as many as 16.2% individuals with no signs of central DI, but in our population all those patients had prior resection of large pituitary tumours. On the other hand, 9 control subjects with normal pituitary function and MR images had well visible hyperintensity of posterior pituitary. Majority of but not all patients with primary psychogenic polydipsia and healthy people would have obvious posterior pituitary bright spot (6). It could be diminished or lost particularly in elderly subjects (19) but according to some present views the high frequency of absent bright spot in healthy individuals (10-20%) could be an overestimate resulting from the technical conditions of MRI 20 years ago (5). Nevertheless, MRI is probably more useful tool for ruling out than for ruling in a diagnosis of central DI (6).
Other aspect that should be examined in MR results in patients with diabetes insipidus are the absence or presence of tumoral or infiltrating lesions, the estimation of pituitary stalk diameter and anterior pituitary height, and the comparison of pre-contrast and post-gadolinium images.
Finding of pituitary stalk thickening is especially important for final diagnosis and treatment because PST could be caused by various, sometimes severe conditions such as tumours or infiltrative processes (20). Langerhans’ cell histiocytosis and germinomas should be considered particularly in children and young adults, while neoplastic (benign and malignant) etiologies of ST is more typical for adults (21). Lymphocytic hypophisitis is more frequently found in patients with other autoimmune diseases. Other processes such as sarcoidosis, Wegener’s granulomatosis and congenital lesions should always be taken into consideration. Establishing the final diagnosis is frequently very challenging and MRI characteristic are often nonspecific. Thus, the cause of PST in high proportion of patients remains unidentified (21). Such patients with the diagnosis of idiopathic DI should be routinely and frequently followed-up.
Conclusions
We conclude that MR imaging of the posterior pituitary lobe is very useful in evaluating the functional status of the neurohypophyseal system. The absence of posterior pituitary hyperintensity correlates well with the presence of central diabetes insispidus and in most cases it could differentiate between central DI patients and healthy individuals and those with psychogenic polydipsia. MR imaging is especially helpful in establishing the diagnosis in new cases of polydipsia-polyuria syndrome but even more important is a detailed medical history and physical examination. Together they could allow finding DI etiology in most cases. We observed less cases of idiopathic DI than it was described in the literature and majority of studied group had DI secondary to prior neurosurgical treatment for large sellar and parasellar tumours. Piśmiennictwo
1. Witek P, Zgliczyński W: Choroby tylnego płata przysadki. [W:] Zgliczyński W (red.): Wielka Interna: Endokrynologia. Wyd. I, Medical Tribune Polska, Warszawa 2011: 92-98.
2. Maghnie M, Cosi G, Genovese E et al.: Central diabetes insipidus in children and young adults. N Engl J Med 2000; 343(14): 998-1007.
3. Chaudhary V, Bano S: Imaging of pediatric pituitary endocrinopathies. Indian J Endocrinol Metab 2012 Sep; 16(5): 682-691.
4. Kurokawa H, Fujisawa I, Nakano Y et al.: Posterior lobe of the pituitary gland: correlation between signal intensity on T1-weighted MR images and vasopressin concentration. Radiology 1998 Apr; 207(1): 79-83.
5. Garel C, Léger J: Contribution of magnetic resonance imaging in non-tumoral hypopituitarism in children. Horm Res 2007; 67(4): 194-202.
6. Fenske W, Allolio B: Clinical review: Current state and future perspectives in the diagnosis of diabetes insipidus: a clinical review. J Clin Endocrinol Metab 2012; 97(10): 3426-3437.
7. Makaryus AN, McFarlane SI: Diabetes insipidus: diagnosis and treatment of a complex disease. Cleve Clin J Med 2006 Jan; 73(1): 65-71.
8. Toumba M, Stanhope R: Morbidity and mortality associated with vasopressin analogue treatment. J Pediatr Endocrinol Metab 2006 Mar; 19(3): 197-201.
9. Di Iorgi N, Napoli F, Allegri AE et al.: Diabetes insipidus – diagnosis and management. Horm Res Paediatr 2012; 77(2): 69-84.
10. Catli G, Abaci A, Demir K et al.: Clinical profile and etiologies of children with central diabetes insipidus: a single-center experience from Turkey. J Pediatr Endocrinol Metab 2012; 25(5-6): 499-502.
11. Pratheesh R, Swallow DM, Rajaratnam S et al.: Incidence, predictors and early post-operative course of diabetes insipidus in paediatric craniopharygioma: a comparison with adults. Childs Nerv Syst 2013; 29(6): 941-949.
12. Kim E: Symptomatic Rathke cleft cyst: clinical features and surgical outcomes. World Neurosurg 2012; 78(5): 527-534.
13. Modan-Moses D, Weintraub M, Meyerovitch J et al.: Hypopituitarism in langerhans cell histiocytosis: seven cases and literature review. J Endocrinol Invest 2001; 24(8): 612-617.
14. Rivera JA: Lymphocytic hypophysitis: disease spectrum and approach to diagnosis and therapy. Pituitary 2006; 9(1): 35-45.
15. Komninos J, Vlassopoulou V, Protopapa D et al.: Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab 2004; 89(2): 574-580.
16. Guitelman M, Garcia Basavilbaso N, Vitale M et al.: Primary empty sella (PES): a review of 175 cases. Pituitary 2013; 16(2): 270-274.
17. Krahulik D, Zapletalova J, Frysak Z, Vaverka M: Dysfunction of hypothalamic-hypophysial axis after traumatic brain injury in adults. J Neurosurg 2010; 113(3): 581-584.
18. Hannon MJ, Orr C, Moran C et al.: Anterior hypopituitarism is rare and autoimmune disease is common in adults with idiopathic centraldiabetes insipidus. Clin Endocrinol (Oxf) 2012; 76(5): 725-728.
19. Brooks BS, el Gammal T, Allison JD, Hoffman WH: Frequency and variation of the posterior pituitary bright signal on MR images. AJR Am J Roentgenol 1989; 153(5): 1033-1038.
20. Leger J, Velasquez A, Garel C et al.: Thickened pituitary stalk on magnetic resonance imaging in children with central diabetes insipidus. J Clin Endocrinol Metab 1999; 84(6): 1954-1960.
21. Turcu AF, Erickson BJ, Lin E et al.: Pituitary stalk lesions: the Mayo Clinic experience. J Clin Endocrinol Metab 2013; 98(5): 1812-1818.
otrzymano/received: 2013-09-17 zaakceptowano/accepted: 2013-10-30 Adres/address: *Izabella Czajka-Oraniec Department of Endocrinology Medical Center of Postgraduate Education Bielański Hospital ul. Cegłowska 80, 01-809 Warszawa tel.: +48 (22) 569-01-05 e-mail: iczajka@cmkp.edu.pl Artykuł Obrazowanie okolicy przysadkowo-podwzgórzowej przy użyciu rezonansu magnetycznego u pacjentów z moczówką prostą pochodzenia centralnego w Czytelni Medycznej Borgis. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








