|
© Borgis - Postępy Nauk Medycznych 10, s. 694-700
*Katarzyna Kubiak-Balcerewicz1, Urszula Fiszer1, Ewa Nagańska1, Cezary Siemianowski1, Aleksander Sobieszek1, Agnieszka Witak-Grzybowska2, Aldona Kosińska-Szot2
Badanie perfuzyjne tomografii komputerowej głowy w diagnostyce przyczyn ostrych ogniskowych objawów neurologicznych – opis czterech przypadków
Perfusion computed tomography in the diagnosis of acute focal neurological symptoms – a report of four cases
1Department of Neurology and Epileptology, Medical Centre of Postgraduate Education,
Professor Witold Orłowski Independent Public Clinical Hospital, Warszawa Head of Department: prof. Urszula Fiszer, MD, PhD 2Department of Roentgenodiagnostics, Professor Witold Orłowski Independent Public Clinical Hospital, Warszawa Head of Department: Agnieszka Witak-Grzybowska, MD Streszczenie
Badanie perfuzyjne tomografii komputerowej (Perf-TK) głowy jest wykorzystywane w ocenie pacjentów z udarem niedokrwiennym mózgu, pojedyncze doniesienia wskazują jednak, że może ono błędnie identyfikować chorych z objawami ubytkowymi w przebiegu napadów padaczkowych. Przedstawiamy opis czterech przypadków chorych z ostrymi ogniskowymi objawami neurologicznymi, bez świeżych zmian niedokrwiennych w rutynowej tomografii komputerowej (TK) głowy, którzy w ciągu 12 godzin od wystąpienia objawów mieli wykonane Perf-TK głowy i badanie elektroencefalograficzne. U pacjentów ostatecznie rozpoznano: udar mózgu niedokrwienny wtórnie ukrwotoczniony (przypadek 1), niedowład ponapadowy Todda (przypadek 2), niedowład w przebiegu niedrgawkowego stanu padaczkowego (przypadek 3) oraz udar mózgu niedokrwienny z towarzyszącym niedrgawkowym stanem padaczkowym (przypadek 4). Uzyskane wyniki wskazują, że Perf-TK głowy może różnicować udar i napady padaczkowe jako przyczynę ostrych ogniskowych objawów neurologicznych. Słowa kluczowe: udar, niedowład Todda, Perf-TK głowy
Summary
Perfusion computed tomography (PCT) is used for acute stroke evaluation, single reports suggest, that it may also falsely identify patients with neurological deficits related to seizures. We report four cases of patients with acute focal neurological symptoms, without ischemic focus in routine computed tomography (CT), who underwent PCT and electroencephalography within 12 hours after symptom’s onset. Patients have finally been diagnosed with ischemic stroke with secondary hemorrhage (case 1), postictal Todd’s paresis (case 2), hemiparesis in course of seizure (case 3) and ischemic stroke with concomitant nonconvulsive status epilepticus (case 4). The results suggest, that PCT may be significant in the differential diagnosis of acute focal neurological symptoms. Key words: stroke, Todd’s paresis, PCT
Introduction
Perfusion computed tomography (PCT) is a recognized tool in an early ischemic stroke imaging. It gives the possibility of visualizing the area of penumbra which, according to some authors, may allow introduction of thrombolytic treatment, even in patients with excluding SITS-MOST criteria (1). There have only few reports been published on the role of this imaging tool in the diagnostics of acute focal neurological symptoms related to seizures. Single reports suggest that it may falsely identify ischemic stroke in these patients while others indicate its role in differentiating these two pathologies. Hand et al. (2) reported, that conditions mimicking stroke may concern up to 31% of patients, in 21% of which seizures are a final cause of symptoms. Since these symptoms may not be possible to differentiate clinically, an effective diagnostic tool would allow avoiding unnecessary, potentially dangerous, thrombolytic treatment. Simultaneously correct antiepileptic treatment could be introduced, which may be particularly significant in cases of undiagnosed status epilepticus. We present four patients with acute focal deficits, without new focal lesions in routine computed tomography (CT), who had PCT and electroencephalography (EEG) performed within 12 hours since the symptoms occurrence. On the third day since the clinical symptoms occurrence we performed a follow-up non-contrast CT in order to visualize presumptive ischemic focus. Patients’ consent to additional procedures was gained according to rules accepted by The Bioethics Committee of Medical Centre of Postgraduate Education. Perfusion parameters in all patients were measured at the level of the basal ganglia, the thalamus and the third ventricle. We analyzed the following parameters: CBF (cerebral blood flow), CBV (cerebral blood volume), MTT (mean transit time). Perfusion parameters were set up as asymmetry indices from corresponding regions of brain hemispheres. We calculated the index of asymmetry (AI) form the formula: AI = (symptomatic hemisphere – reference hemisphere)/(symptomatic hemisphere + reference hemisphere) x 100% (3). On the basis of literature (3) as a hypoperfusion or hyperperfusion we assumed the asymmetry index above 10%.
Cases REPORTS
The cases are presented together in a form of a table (tab. 1, fig. 1-4).
Table 1. Cumulative characteristics of presented cases.
*NIHSS – National Health Institutes Stroke Scale.
**Arrows indicate changes in perfusion parameters in symptomatic hemisphere in comparison to the contralateral hemisphere. Other abbreviations are explained in the introduction. 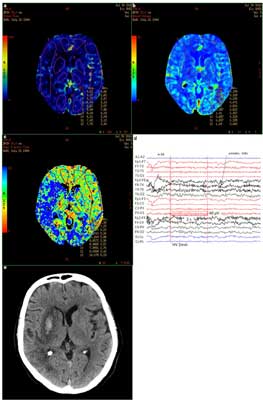 Fig. 1. PCT (A – CBF, B – CBV, C – MTT) and EEG (D) within 12 hours since the symptoms occurre, as well as non-contrast CT on the third day symptoms onset (E) in a patient with ischemic stroke with secondary hemorrhagic transformation (case 1). Description in the text.
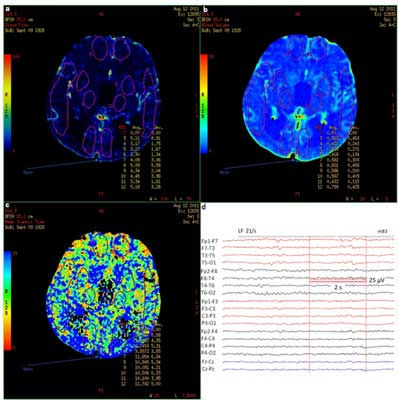 Fig. 2. PCT (A – CBF, B – CBV, C – MTT) and EEG (D) within 12 hours since the symptoms occurre in a patient with Todd’s paresis (case 2). Description in the text.
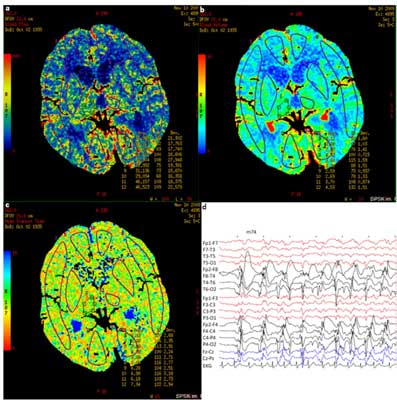 Fig. 3. PCT (A – CBF, B – CBV, C – MTT) and EEG (D) within 12 hours since the symptoms occurred in a patient in a nonconvulsive status epilepticus with coexisting paresis of right limbs (case 3). Description in the text.
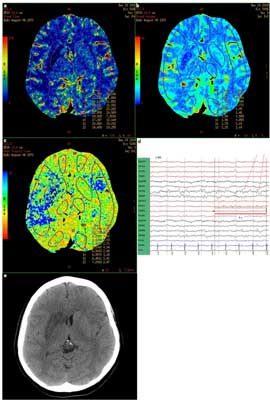 Fig. 4. PCT (A – CBF, B – CBV, C – MTT), EEG (D) within 12 hours since the symptoms occurred as well as non-contrast CT on the third day sience symptoms onset (E) in a patient with ischemic stroke with nonconvulsive status epilepticus (case 4). Description in the text.
Discussion
Even though a transient motor deficit following epileptic seizure (Todd’s paresis) has been described in the literature since 1827, until now no one could interchangeably state its etiopathogenesis. While analyzing the available data we can assume that there are at least two separate pathophysiological mechanisms. The first one concerns the actual paresis after seizure, which occurs after demission of bioelectric activity characteristic for the seizure and should be accompanied by focal slowing in the basic activity in EEG. It could be caused by “exhaustion” of neurons secondary to hypoxia, the increase in lactates and local disturbances in blood flow (4, 5), what could result in focal hypoperfusion of the brain tissue. This conception, although often undermined, finds confirmation in some of the newer reports that explain the reduction of cerebral blood flow after seizure, to be a reflexive reaction following decreased brain oxygen and glucose metabolism (6). Hypoperfusion after seizure was found in many single photon emission computed tomography studies (SPECT), however, some patient with temporal epilepsy had also hyperperfusion lasting up to 30 minutes in the hippocampal region (7). Moreover, another study using perfusion magnetic resonance showed hippocampal hypoperfusion after seizure, but hyperperfusion in the parahippocampal gyrus as a result of increased metabolism in order to preserve the appropriate threshold of neuron excitability (8).
Until now there have been only a few publications with PCT results in patient with focal deficits after seizures. They indicate mainly the presence of hypoperfusion after seizures in the hemisphere with the source of epileptic discharges, but except for basal ganglia (9) or the white matter (10), exceeding traditional vascular territories. Gelfland et al. (9) described 8 patients with focal hypoperfusion, 1 with hyperperfusion and 1 with isolated prolonged MTT, however, in some patients no EEG was performed. Hauf et al. (3) included 3 patients with paresis after seizure in their research, in all of which hypoperfusion was found. In both these research, in cases of hypoperfusion, perfusion parameters were similar to those in the ischemic stroke – CBF and CBV decreased with increased MTT. However, in one case there was hypoperfusion with decreased CBF and CBV with no changes in MTT (10). MTT is a sensitive index of hypoperfusion secondary to a large vessel stenosis, that is why it’s relative symmetry would rather indicate a metabolic cause of symptoms (10) and may at the same time be a differentiating parameter. In our case of Todd’s paresis [2] MTT did not change significantly, but we visualized hyperperfusion of left hemisphere (frontal and occipital lobe and the basal ganglia) with maximum AI 16% with decrease in record amplitude and predominance of slow waves over the left hemisphere in EEG. According to our knowledge such case hasn’t been published yet. We cannot, however exclude that during neuroimaging there was a transient epileptic activity, but we did not observe any changes in patient’s state condition at that time. Moreover, localization of hyperperfusion areas did not indicate the stroke in the phase of reperfusion. Less likely, but not impossible, would be the presence of multifocal vascular malformations that could be the reason for increased blood flow. Finally, retrospectively analyzed course of the disease did not raise any suspicion of encephalitis or migraine.
We used the conception of intra-seizure limb immobility (11), while making the diagnosis in the third case raport. It comes from Gowers’s considerations (12) who, not finding the dependence between the time of seizure and paralysis duration, postulated the mechanism of active retardation. Impulses from basal ganglia would have a modulating influence on retarding interneurons in the motor cortex (13). Luders et al. (14) proved that additional motor field of both hemispheres stimulation causes retardation of precise movements. However, there was a patient with paresis during seizure described who did not have epileptic discharges in the additional motor field registered, while they were present in the motor cortex (15).
Numerous SPECT and MR studies confirm the presence of focal hyperperfusion during focal seizure, which by many authors is explained by the increase in the brain metabolism. There is also an alternative conception of hyperperfusion mechanism concerning neuroprotective factors release as a reaction to epileptic discharges, such as vascular endothelial growth factor which, by nitric oxide, causes vessel dilating (16). However, there was also hypoperfusion during seizure described which occurrence was explained as “stealing phenomenon” from other brain regions to which epileptic discharges propagate (17).
The case we described [3] was diagnosed as the paresis during seizure, in spite of the fact that the bioelectrical features of status epilepticus in EEG were expressed mainly over the hemisphere ipsilateral to clinical symptoms. We found features of hyperperfusion in left occipital lobe (AI: 13.6%), but only in CBV. Although yet known PCT studies in patients with paresis during seizures also indicate hyperperfusion, but it concerns only the region of the hemisphere with epileptic discharges. Analogically to hypoperfusion after seizure hyperperfusion during seizure described in the literature manifested in the increase in CBF and CBV and decrease in MTT (3, 18-20). Only one case report indicates lack of changes in MTT symmetry with accurate increase in CBF and CBV (21). Hyperperfusion was also found in the patient in whom the region of post-infarction scar was the source of epileptic discharges, despite the fact that theoretically in this region we would rather suppose to find hypoperfusion (19). In the group of 9 patients with nonconvulsive status epilepticus, hyperperfusion avoided the white matter, mean AI (CBV) was 21%, but the lack of hyperperfusion did not exclude nonconvulsive status epilepticus (3). It is difficult to explain hyperperfusion which we found in the occipital lobe contralateral to epileptic discharges in EEG. We cannot exclude that the change in a single perfusion parameter could be a result of unrecognized vascular malformation. Finally, small asymmetry index as well as unexpected prolonged MTT in this area suggest that these changes may be accidental and result from the technical limitations of the method.
Stroke hypoperfusion found in PCT is widely described in the literature. The case we presented [1] is typical with decreased CBV and CBF and increased MTT. AI values in regions, where in the third day since symptoms onset ischemic focus with secondary hemorrhage occurred, were between 24 and 42%. It is noticeable, that in the lateral part of the right temporal lobe there is no decrease in CBV but there is lengthening of MTT and decrease in CBF – this area is mostly excluded from the ischemic area visible on the third day since symptoms onset and is very likely corresponding to penumbra.
Another diagnostic challenge are patients with ischemic stroke and concomitant seizures. Stroke begins with seizures in about 7% of patients (18). The case [4] shows focal hypoperfusion corresponding to ischemia revealed in a follow-up CT, apart from paroxysmal activity found in these regions in EEG. Asymmetry indices (AI) were slightly lower than in case number 3 (14 – 26%), despite patient’s more severe neurological state (NIHSS 17 vs. 14 points). This type of changes in perfusion parameters, according to above mentioned literature data could also occur in paresis after seizure without a stroke, however, the diagnosis of stroke could be suggested mainly by the localization of hypoperfusion typically in the medial cerebral artery vascularization territory and inclusion of the white matter. In such cases, the potential role of PCT in differentiation is particularly interesting, since, according to Polish Society of Neurology 2012 guidelines, stroke that begins with seizures is no longer an absolute contraindication to thrombolytic treatment (22).
The gained results indicate that PCT may differentiate ischemic stroke and seizures as the cause of acute focal neurological symptoms. On the basis of described cases it seems that the differentiating factor could be the presence or the lack of hypoperfusion in the symptomatic hemisphere. It is necessary to collect the appropriate group of patients in order to perform statistical analysis and get further conclusions. Piśmiennictwo
1. Cortijo E, Calleja AI, Garcia-Bermejo P et al.: Perfusion computed tomography makes it possible to overcome important SITS-MOST exclusion criteria for the endovenous thrombolysis of cerebral infarction. Rev Neurol 2012; 54: 271-276.
2. Hand P, Kwan J, Lindley RI et al.: Distinguishing between stroke and mimic at the bedside: The Brain Attack Study. Stroke 2006; 37: 769-775.
3. Hauf M, Slotboom J, Nirkko A et al.: Cortical regional hyperperfusion in nonconvulsive status epilepticus measured by dynamic brain perfusion CT. Am J Neuroradiol 2009; 30: 693-698.
4. Yarnell PE: Todd’s paralysis: a cerebrovascular phenomenon? Stroke 1975; 6: 301-303.
5. Efron R: Post-epileptic paralysis: theoretical critique and report of a case. Brain 1961; 84: 381-394.
6. McNamara JO: Cellular and molecular basis of epilepsy. J Neurosci 2002; 14: 3413-3425.
7. Newton MR, Berkovic SF, Austin MC et al.: Postictal switch in blood flow distribution and temporal lobe seizures. J Neurol Neurosurg Psychiatry 1992; 55: 891-894.
8. Leonhardt G, De Greiff A, Weber J et al.: Brain perfusion following single seizures. Epilepsia 2005; 46: 1943-1949.
9. Gelfland JM, Wintermark M, Josephson SA: Cerebral perfusion-CT patterns following seizure. Eur J Neurol 2010; 17: 594-601.
10. Mathews MS, Smith WS, Wintermark M et al.: Local cortical hypoperfusion imaged with CT perfusion during postictal Todd’s paresis. Neuroradiology 2008; 50: 397-401.
11. Oestreich LJ, Berg MJ, Bachmann DL et al.: Ictal contralateral paresis in complex partial seizures. Epilepsia 1995; 36: 671-675.
12. Gowers WR: Epilepsy and other chronic convulsive diseases: their causes, symptoms and treatment. J & A Churchill, 1st ed., London 1881: 1-309.
13. Hanajima R, Ugawa Y, Terao Y et al.: Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders. J Neurol Sci 1996; 140: 109-116.
14. Luders H, Lesser RP, Dinner DS et al.: The second sensory area in humans: evoked potential and electrical stimulation studies. Ann Neurol 1985; 17: 177-184.
15. Matsumoto R, Ikeda A, Ohara S et al.: Nonconvulsive focal inhibitory seizure: subdural recording from motor cortex. Neurology 2000; 55: 429-431.
16. Ahmad S, Hewett PW, Wang P et al.: Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric-oxide mediated angiogenesis. Circ Res 2006; 99: 715-722.
17. Lee HW, Hong SB, Tae WS: Opposite ictal perfusion patterns of subtracted SPECT hyperperfusion and hypoperfusion. Brain 2000; 123: 2150-2159.
18. Masterson K, Vargas MI, Delavelle J: Postictal deficit mimicking stroke: Role of perfusion CT. J Neuroradiol 2009; 36: 48-51.
19. Royter V, Paletz L, Waters MF: Stroke vs. status epilepticus. A case report utilizing CT perfusion. J Neurol Sci 2008; 266: 174-176.
20. Guerrero WR, Dababneh H, Eisenschenk S: The role of perfusion CT in identifying stroke mimics in the emergency room: a case of status epilepticus presenting with perfusion CT alterations. Int J Emerg Med 2012; 5: 4.
21. Lie C-H, Seifert M, Poggenborg J et al.: Perfusion computer tomography helps to differentiate seizure and stroke in acute setting. Clin Neurol Neurosurg 2011; 113: 925-927.
22. Wytyczne Grupy Ekspertów Sekcji Chorób Naczyniowych Polskiego Towarzystwa Neurologicznego. Neurol Neurochir Pol 2012; 46 (suppl. 1): 25.
otrzymano/received: 2013-07-17 zaakceptowano/accepted: 2013-09-04 Adres/address: *Katarzyna Kubiak-Balcerewicz Department of Neurology and Epileptology Medical Centre of Postgraduate Education Professor Witold Orłowski Independent Public Clinical Hospital ul. Czerniakowska 231, 00-416 Warszawa tel.: +48 (22) 629-43-49; fax: +48 (22) 584-13-06 e-mail: katarzyna.izabela@gmail.com Artykuł Badanie perfuzyjne tomografii komputerowej głowy w diagnostyce przyczyn ostrych ogniskowych objawów neurologicznych – opis czterech przypadków w Czytelni Medycznej Borgis. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








