|
© Borgis - Postępy Nauk Medycznych 10, s. 678-682
*Urszula Fiszer1, Marta Piaścik-Gromada1, Marta Jethon1, Małgorzata Michałowska1, Katarzyna Bocian2, Tomasz Szatanowski1, Grażyna Korczak-Kowalska2, 3
Ekspresja transportera dopaminy w limfocytach krwi obwodowej w chorobie Parkinsona
Dopamine transporter immunoreactivity in peripheral blood lymphocytes in Parkinson’s disease**
1Department of Neurology and Epileptology, Medical Centre of Postgraduate Education, Warszawa
Head of Department: prof. Urszula Fiszer, MD, PhD 2Department of Immunology, Faculty of Biology, University of Warsaw Head of Department: prof. Grażyna Korczak-Kowalska, PhD 3Department of Clinical Immunology, Transplantation Institute, Medical University, Warszawa Head of Department: prof. Andrzej Górski, MD, PhD Streszczenie
Wstęp. Limfocyty krwi obwodowej służą jako model do badań zmian układu neuroprzekaźnik-receptor. Cel pracy. Celem pracy było badanie ekspresji transportera dopaminy (DAT) w limfocytach krwi obwodowej pacjentów z chorobą Parkinsona (PD) w porównaniu do grupy kontrolnej oraz wpływu różnych parametrów. Materiał i metody. Do badania włączono trzydziestu czterech pacjentów z PD, a także piętnaście osób cierpiących z powodu dyskopatii, incydentów niedokrwienia lub zawrotów głowy. Analiza odsetka oraz ekspresji zewnątrzkomórkowej (GeoMean) DAT na komórkach CD45+ przeprowadzono przy użyciu cytometrii przepływowej. Wyniki. Nie znaleziono różnicy pomiędzy grupą pacjentów z PD oraz grupą kontrolną dla odsetka komórek CD45+ DAT+, a także dla ekspresji zewnątrzkomórkowej DAT na komórkach CD45+. Znaleziono pozytywną korelację pomiędzy ekspresją zewnątrzkomórkową DAT na komórkach CD45+ a wiekiem początku wystąpienia objawów PD, a także pomiędzy ekspresją zewnątrzkomórkową DAT na komórkach CD45+ a wiekiem chorych. Badanie nie wykazało korelacji pomiędzy ekspresją zewnątrzkomórkową DAT na komórkach CD45+ a długością choroby ani w odniesieniu do czasu trwania, ani dawki lewodopy. Wnioski. Cytometria przepływowa jest użytecznym narzędziem badawczym ekspresji DAT w limfocytach. Słowa kluczowe: transporter dopaminy, choroba Parkinsona, cytometria przepływowa, limfocyty obwodowej krwi obwodowej
Summary
Introduction. Peripheral blood lymphocytes provide a model to study the changes of neurotransmitter-receptor systems. Aim. The aim of the study was to investigate the dopamine transporter (DAT) immunoreactivity in peripheral blood lymphocytes of Parkinson’s disease (PD) patients as compared to controls and impact of various parameters. Material and methods. Thirty four PD patients were included in the study, as well as fifteen controls who suffered from discopathy, ischemic events or vertigo. The analysis of the percentage and the expression of extra-cellular (GeoMean) anti-DAT in CD45+ cells was performed using flow cytometry. Results. No differences between groups of PD patients and controls were found for percentage of CD45+ DAT+, neither for expression of extra-cellular anti-DAT in CD45+. We found positive correlation between the expression of extra-cellular anti-DAT and age of clinical onset of PD, either the expression of extra-cellular anti-DAT and the age of PD patients. The study showed no correlation between the expression of extra-cellular anti-DAT and the duration of the disease, neither with respect to the duration or dose of the levodopa treatment. Conclusion. Flow cytometric analysis of DAT expression in lymphocytes provide useful tool for research. Key words: dopamine transporter, Parkinson’s disease, flow cytometry, peripheral blood lymphocytes
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder with a marked reduction of striatal dopamine (DA) concentration. The dopamine transporter (DAT) is a presynaptic membrane protein expressed in DA-synthesizing neurons. It clears dopamine released into the extra-cellular space, thereby regulating the amplitude and duration of DA signaling. Altered DAT expression may represent a response to pathological processes, and changes in DAT can be used to monitor disease progression and response to treatment (1-3). Imaging of DAT by single photon emission CT (SPECT), or positron emission tomography (PET) proved to be practical for identifying presynaptic parkinsonism, however, is not widely available due to its high costs (4, 5).
Peripheral blood lymphocytes (PBL) provide a model to study the changes of neurotransmitter-receptor systems in neurological diseases (6). The DAT is expressed in PBL such as lymphocytes and platelets. Initial studies show a significant loss of DAT immunoreactivity in PD patients in the early clinical stages of the disease (7, 8). The pathophysiology of DAT expression in PBL in PD is rather obscure.
Aim
The aim of the study was to investigate the DAT immunoreactivity in PBL of PD subjects compared to controls and impact of various parameters (age of the subject, age of the clinical onset of PD, duration of the disease, the duration and dose of the levodopa treatment).
We applied flow cytometric analysis for DAT expression in PBL. It may represent an improvement with respect to the immunocytochemical metods applied so far by others (7, 8).
Material and methods
Patients population
The inclusion of PD patients in the study was based on the clinical criteria set forth according to UK PD Society Brain Bank criteria (9) and the duration of the disease lasting for at least 3 years.
Forty nine patients have been included in the study. The PD patients (34 persons) were divided into 3 groups: A) 3-5 years from the onset of PD (12 persons); B) more than 5 years from the onset of PD without motor complication after levodopa treatment (11 persons); C) more than 5 years from the onset of PD with motor complication after the levodopa treatment (11 persons). Controls (15 persons) suffered from discopathy, ischemic events or vertigo.
The progression of PD disease was evaluated using Hoehn and Yahr scale and the Unified Parkinson’s Disease Rating Scale (UPDRS) part I, II and III, while the cognitive functions were evaluated using Mini Mental State Examination (MMSE) and the mood using the Montgommery-Åsberg Depression Rating Scale (MADRS).
Flow cytometric analysis
Flow cytometric analysis was applied to measure DAT immunoreactivity in PBL.
Mononuclear cells (PBMC) were isolated from the peripheral blood of patients (tubes contained EDTA) by density-gradient centrifugation using gradisol-L (Aqua Medica Łódź). The cells were washed and resuspended at 1*106 cells/100 μl in CellWash with 0.5% BSA (Becton Dickinson). To determine the expression of extra-cellular anti-DAT in CD45+ cells, the PBMC were labeled with monoclonal antibody against CD45 (anti-CD45-PE) marker (Becton Dickinson) and with optimal dilution of polyclonal antibodies: anti-DAT extracellular Loop 2 (SIGMA), as well as with respective isotype control antibodies. As secondary antibody was used FITC-conjugated F(ab’)2 fragment (SIGMA).
The analysis of the percentage and expression of extra-cellular anti-DAT in CD45 cells was performed using FACSCalibur (Becton Dickinson) and Cell Quest software. The lymphocytes were specifically analyzed by selective gating based on the parameters of forward and side scatter. The results were based on the analysis of at least 10.000 cells and were shown as the percentage of positively labeled cells (fig. 1).
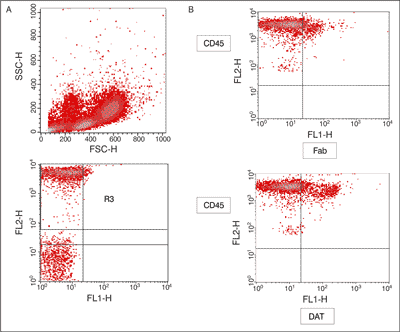 Fig. 1. The dot plots show representative data illustrating the analysis methods of dopamine transporter (DAT) + cells among CD45+ cells.
A – the dot plot show the forward scatter (FSC/SSC) distribution and the gate (region R1) used to select lymphocytes for analysis the R1 gated events were then analyzed for CD45 PE staining, and positive cells were gated (region R2), the final dot plot was established by combined gating of events using R1 and R2; B – the analysis the percent and expression of extra-cellular anti-dopamine transporter (DAT) in CD45+ cells. Statistical analysis
The data were normally distributed (calculated using Kolmogorov-Smirnov test). T-Student test, chi-square test and Pearson’s linear correlation analysis were used. The statistical significance was accepted at p < 0.05.
Results
Clinical characteristics of the groups
The clinical and demographic data of all the groups have been presented in table 1. There was no difference in sex and age between the groups of PD patients.
Table 1. Clinical and demographic data of patients (Parkinson’s disease – PD, and controls).
mean values ± SD; group A – 3-5 years from onset of PD; group B – more than 5 years from onset of PD without motor complication after levodopa treatment; group C – more than 5 years from onset of PD with motor complication after levodopa treatment; group D – controls; ns – non statistically significant
The results are showed in the table 2. No differences between groups of PD patients and controls were found for percent of CD45+ DAT+, neither for expression of extra-anti-DAT in CD45+. Only a tendency to decrease an expression of extra-anti-DAT in CD45+ cells was found in group of all PD patients compared to controls.
We found positive correlation between expression of extra-cellular anti-DAT and age of clinical onset of PD (Pearson correlation r = 0.37; p < 0.04) (fig. 2), as well as between expression of extra-cellular anti-DAT and age of PD patients (Pearson correlation r = 0.35; p < 0.05) (fig. 3), but not for controls (r = -0.20, ns). The study showed no correlation between expression of extra-cellular anti-DAT and duration of disease, neither with respect to the duration nor dose of the levodopa treatment.
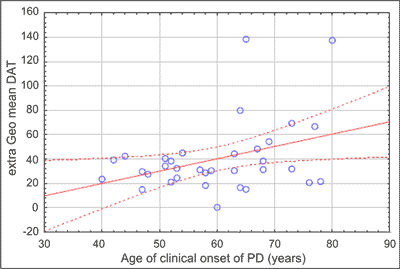 Fig. 2. Correlation between expression of extra-cellular anti-dopamine transporter (DAT) and age of clinical onset of Parkinson’s disease (PD) (all PD patients – n = 34), Pearson correlation 0.35; p < 0.05.
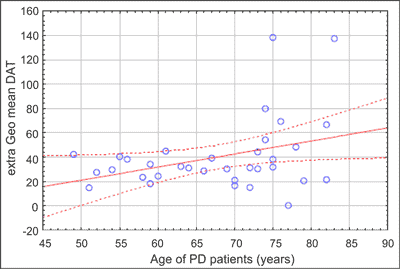 Fig. 3. Correlation between expression of extra-cellular anti-dopamine transporter (DAT) and age of Parkinson‘s disease (PD) patients (all PD patients – n = 34), Pearson correlation 0.37; p < 0.04.
Discussion
Prior studies showed the a significant loss of DAT immunoreactivity in PD drug-native and dopamine agonist-treated patients (mean age 54 ± 6 years) in the early clinical stages of the disease compared to healthy subjects (mean age 53 ± 5 years) (7). In our study only a tendency to decrease an expression of extra-anti-DAT in CD45+ cells was found in groups of PD patients compared to controls (tab. 2). No difference between the group of patients with and without motor fluctuation had been found.
Table 2. Dopamine transporter (DAT) immunoreactivity in blood lymphocytes in groups of examined patients (Parkinson’s disease – PD, and controls).
group A – 3-5 years from onset of PD; group B – more than 5 years from onset of PD without motor complication after levodopa treatment; group C – more than 5 years from onset of PD with motor complication after levodopa treatment; group D – controls; extra % CD45+ DAT+ – percent of extra-cellular anti-dopamine transporter in CD45+ cells; extra Geo mean DAT – expression of extra-cellular anti-dopamine transporter; mean values ± SD; ns – non statistically significant
The application of flow cytometric analysis for DAT expression in PBL is considered as a significant improvement with respect to the immunocytochemical metods applied so far by others (7, 8). The results were based on analysis of at least 10.000 cells. This technique has shown to be quite useful, and simple in practice (albeit expensive). This work may be replicated by others.
Barili et al. (10) suggested age-dependent reduction of dopaminergic function occurs in human PBL, particulary a decline in the density of lymphocyte dopamine D3 receptor. Research projects concerning aging effects on DAT expression and compensatory mechanisms are in progress (11, 12).
Studies of DAT genotype-phenotype associations have not clarified the biological mechanism of DAT regulation (13-15).
The study showed positive correlation between the expression of extra-cellular anti-DAT and the age of PD patients (Pearson correlation r = 0.35; p < 0.05). It has been evidenced that there is an enhanced DAT activity in middle-ages Gdnf heterozygous mice (16), and recently reported that striatal DAT binding correlates with serum brain derived neurotrophic factor levels in patients with PD (17). Genomewide association studies for onset age are in PD in progress (18-20).
The study showed no positive correlation between the expression of extra-cellular anti-DAT and the duration of disease, neither with respect to the duration nor dose of the levodopa treatment. There is a limitation regarding the above study, unfortunately, we did not have a possibility to compare the above results with the group of untreated PD patients, because our team was unable to identify such a group. The possible alteration of DAT binding by effects of drugs remains a concern (21). Pre-clinical animal studies investigating DAT expression, and whether dopaminergic drugs might influence the expression of DAT are in progress (22-24). The modulation of expression of the DAT by dopaminergic drugs was investigated by flow cytometry in PD patients (25); the results demonstrated that levodopa was different from dopamine agonist in its regulation of DAT expression in PBL, but exposure to levodopa did not modify the PBL DAT mean fluorescence intensity.
A question whether the flow cytometric analysis for DAT expression in PBL may be used as a marker in the assessment of the PD progress remains still open. Such views have been already presented: “DAT in patients with different neuropsychiatric disorders as a peripheral marker of the same brain structure is just emerging...” (6).
The results of our study contribute to current knowledge concering DAT in PBL and provide useful tool for further research of PD.
**This work was presented on Congress of the European Federation of Neurological Societies EFNS in Florence, 12-15 September 2009. Abstracts of the 13th EFNS, European Journal of Neurology 2009; 16 (suppl. 3): 124. This work conducted by the authors was supported by Medical Centre of Postgraduate Education in Warsaw (grant no 501-1-1-13-30/07). Piśmiennictwo
1. Bannon MJ: The dopamine transporter: role in neurotoxicity and human disease. Toxicol Appl Pharmacol 2005; 204: 355-360.
2. Nutt JG, Carter JH, Sexton GJ: The dopamine transporter: importance in Parkinson’s disease. Ann Neurol 2004; 55: 766-777.
3. Storch A, Ludolph AC, Schwarz J: Dopamine transporter: involvement in selective dopaminergic neurotoxicity and degeneration. J Neural Transm 2004; 111: 1267-1286.
4. De la Fuente-Fernandez R, Lu JO, Sossi V et al.: Biochemical variations in the synaptic level of dopamine preced motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neuro 2001; 49: 298-303.
5. Plotkin M, Amthauer M, Quill H et al.: Imaging of dopamine transporters and D2 receptors in vascular parkinsonism: a report of four cases. J Neural Transm 2005; 112: 1355-1361.
6. Marazziti D, Consoli G, Masala I et al.: Latest advancements on serotonin and dopamine transporters in lymphocytes. Mini Rev Med Chem 2010; 10: 32-40.
7. Caronti B, Antonini G, Calderaro C et al.: Dopamine transporter immunoreactivity in peripheral blood lymphocytes in Parkinson’s disease. J Neural Transm 2001; 108: 803-807.
8. Pellicano C, Buttarelli FR, Circella A et al.: Dopamine transporter immunoreactivity in peripheral blood lymphocytes discriminates Parkinson’s disease from essential tremor. J Neural Transm 2007; 114: 935-938.
9. Hughes HJ, Daniel SE, Kilford L et al.: Accuracy of clinical diagnosis of idiopathic Parkinson’s disease a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55: 181-184.
10. Barili P, Bronzetti E, Felici L et al.: Age-dependent changes in expression of dopamine receptor subtypes in human peripheral blood lymphocytes. J Neuroimmunol 1996; 71: 42-50.
11. Cruz-Muros I, Afonso-Oramas D, Abreu P et al.: Aging effects on the dopamine transporter expression and compensatory mechanisms. Neurobiol Aging 2009; 30(6): 973-986.
12. Yue F, Zeng S, Wu D et al.: Age-related decline in motor behavior and striatal dopamine transporter in cynomolgus monkeys. J Neural Transm 2012; 119(8): 943-952.
13. Lee JY, Cho J, Lee EK et al.: Differential genetic susceptibility in diphasic and peak-dose dyskinesias in Parkinson’s disease. Mov Disord 2011; 26: 73-79.
14. Lin C, Liu HC, Tsai SJ et al.: Association study for Parkinson’s disease and a dopamine transporter gene polymorphism (1215A/G). Eur Neurol 2002; 48: 207-209.
15. Shumay E, Fowler JS, Volkow ND: Genomic features of the human dopamine transporter gene and its potential epigenetic States: implications for phenotypic diversity. PLoS One 2010; 5(6): e11067.
16. Littrell OM, Pomerleau F, Huettl P et al.: Enhanced dopamine transporter activity in middle-aged Gdnf heterozygous mice. Neurobiol Aging 2012; 33(2): 427.e1-14.
17. Ziebell M, Khalid U, Klein AB et al.: Striatal dopamine transporter binding correlates with serum BDNF levels in patients with striatal dopaminergic neurodegeneration. Neurobiol Aging 2012; 33(2): 428.e1-5.
18. Booij J, van Amelsvoort T, Boot E: Co-occurrence of early-onset Parkinson disease and 22q11.2 deletion syndrome: Potential role for dopamine transporter imaging. Am J Med Genet 2010; 152: 2937-2938.
19. Panzacchi A, Moresco RM, Garibotto V et al.: A voxel-based PET study of dopamine transporters in Parkinson’s disease: relevance of age at onset. Neurobiol Dis 2008; 31: 102-109.
20. Pankratz N, Wilk JB, Latourelle JC, PSG-PROGENI and GenePD Investigators, Coordinators and Molecular Genetic Laboratories et al.: Genomewide association study for susceptibility genes contributing to familial Parkinson disease. Hum Genet 2009; 124: 593-605.
21. Winogrodzka A, Booij J, Wolers ECh: Disease-related and drug-induced changes in dopamine transporter expression might undermine the reliability of imaging studies of disease progression in Parkinson’s disease. Parkinsonism Relat Disord 2005; 11: 475-484.
22. Fernagut PO, Li Q, Dovero S et al.: Dopamine transporter binding is unaffected by L-DOPA administration in normal and MPTP-treated monkeys. PLoS One 2010; 225(11): e14053.
23. Lehmensiek V, Tan E, Liebau MS et al.: Dopamine transporter – mediated cytotoxicity of 6-hydroxydopamine in vitro depends on expression of mutant α-synucleins related to Parkinson’s disease. Neurochem Int 2006; 48: 329-340.
24. Sotnikova TD, Caron MG, Gainetdinov RR: DDD mice, a novel acute mouse model of Parkinson’s disease. Neurology 2006; 67: S12-S17
25. Fanciulli A, Misasi R, Campanelli D et al.: Dopaminergic drug-induced modulation of the expression of the dopamine transporter in peripheral blood lymphocytes in Parkinson disease. Pharmacol Rep 2011; 63: 1056-1060.
otrzymano/received: 2013-07-17 zaakceptowano/accepted: 2013-09-04 Adres/address: *Urszula Fiszer Department of Neurology and Epileptology Medical Centre of Postgraduate Education ul. Czerniakowska 231, 00-416 Warszawa tel.: +48 (22) 629-43-49; fax: +48 (22) 584-13-06 e-mail: fiszeru@cmkp.edu.pl Artykuł Ekspresja transportera dopaminy w limfocytach krwi obwodowej w chorobie Parkinsona w Czytelni Medycznej Borgis. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








