|
© Borgis - Postępy Nauk Medycznych 10, s. 667-672
Maria Maliszewska1, *Urszula Fiszer1, Witold Palasik1, Michał Morton1, Wiesław Tadeusiak2
Podwyższony poziom troponiny I – czynnik złego rokowania w udarach niedokrwiennych mózgu
Elevated troponin I level – a predictor of poor prognosis after ischemic stroke**
1Department of Neurology and Epileptology, Medical Centre of Postgraduate Education, Warszawa
Head of Department: prof. Urszula Fiszer, MD, PhD 2Department of Anaesthesiology and Intensive Therapy, Medical Centre of Postgraduate Education, Warszawa Head of Department: A.i. Małgorzata Malec-Milewska, MD, PhD Streszczenie
Wstęp. W ostrej fazie udaru niedokrwiennego mózgu (UNM) obserwuje się liczne zaburzenia kardiologiczne, przede wszystkim w zapisie krzywej EKG. Dodatkowym czynnikiem ryzyka wystąpienia zaburzeń kardiologicznych jest udar w obrębie prawej wyspy. Monitorowanie zapisu EKG oraz kontrola poziomu troponiny I (cTnI) mogą być pomocne w rozpoznawaniu tych zaburzeń. Cel pracy. Celem tej pracy było ustalenie, czy podwyższone stężenie cTnI wpływa na przeżycie i prognozę u pacjentów z UNM, ustalenie czynników predysponujących do jego wzrostu oraz określenie korelacji pomiędzy lokalizacją udaru a wzrostem stężenia cTnI. Materiał i metody. Przy przyjęciu u pacjentów z UNM oznaczano stężenie cTnI, wykonywano badanie tomografii komputerowej głowy i badanie EKG z 12 odprowadzeniami. Po miesiącu i sześciu miesiącach od UNM badano ponownie chorego i/lub rejestrowano zgon. Wyniki. Pacjenci z podwyższonym stężeniem cTnI mieli 2,22-krotnie (95% CI 1,14-4,34; p < 0,019) i 2,33-krotnie (95% CI 1,37-3,96; p < 0,002) odpowiednio wyższe ryzyko zgonu w ciągu 30 i 180 dni. Niezależnymi czynnikami ryzyka wpływającymi na wzrost stężenia cTnI były: zaburzenia repolaryzacji (OR = 3,49; 95% CI 1,08-11,28; p < 0,037), dodatkowe pobudzenia komorowe (VEB) (OR = 10,12; 95% CI 1,57-65,03; p < 0,015), wzrost stężenia glukozy (OR = 7,38; 95% CI 2,07-26,37; p < 0,002), ognisko udaru w zakresie prawej tętnicy środkowej mózgu (MCA) (OR = 6,60; 95% CI 1,90-22,93; p < 0,003) i wzrost stężenia białka ostrej fazy (CRP) (OR = 5,90; 95% CI 1,89-18,45; p < 0,002). Wnioski. U pacjentów z UNM występuje istotny wzrost ryzyka pojawienia się zaburzeń kardiologicznych. W identyfikacji tych pacjentów pomóc może pomiar stężenia cTn. Słowa kluczowe: udar niedokrwienny mózgu, troponina, śmiertelność, czynniki
Summary
Introduction. Multiple ECG abnormalities can be observed during the acute phase of ischemic stroke (IS). Right-hemisphere stroke with insular involvement is also a risk factor for cardiac complications. ECG monitoring and levels of cardiac-specific troponin (cTn) may help to identify these disorders. Aim. The aim of this study was to evaluate the influence of elevated concentrations of cTnI on survival and prognosis in patients with IS to identify factors predisposing to cTnI elevation and to determine whether stroke location and elevated cTnI concentration were correlated. Material and methods. The cTnI concentration, CT imaging study and a standard 12-lead ECG were performed on admission in patients with IS. At one and six months after IS, the neurological status was re-evaluated or the death of the patient was registered. Results. Patients with elevated cTnI concentrations had 2.22-fold (95% CI 1.14-4.34; p < 0.019) and 2.33-fold (95% CI on 1.37-3.96; p < 0.002) higher 30- and 180-day mortality risk, respectively. Other independent prognostic factors influencing elevated cTnI concentration were: repolarization abnormalities (OR = 3.49; 95% CI 1.08-11.28; p < 0.037). Ventricular ectopic beats (VEB) (OR = 10.12; 95% CI 1.57-65.03; p < 0.015), increased glucose level (OR = 7.38; 95% CI 2.07-26.37; p < 0.002), lesion in the right MCA perfusion territory (OR = 6.60; 95% CI 1.90-22.93; p < 0.003) and increased CRP concentration (OR = 5.90; 95% CI 1.89-18.45; p < 0.002). Conclusion. There is a significantly increased risk of cardiac complications among patients with IS. Measurement of cTnI concentrations may improve clinical identification of at-risk patients. Key words: ischemic stroke, troponin, mortality, factors
INTRODUCTION
Multiple electrocardiographic (ECG) abnormalities can be observed during the acute phase of ischemic stroke (IS), the most common of which are arrhythmias and repolarization abnormalities with QT interval prolongation and/or ST segment depression. These abnormalities may be transient or may indicate an existing cardiac disorder (1-4). Right-hemisphere stroke with insular involvement is an additional risk factor for cardiac complications of cerebral origin and also for sudden death during this period (5-7). Stimulation of the right insula increases blood pressure and heart rate, whereas stimulation of the left insula causes a decrease in blood pressure and bradycardia (8). Such abnormalities are often observed in patients despite the absence of any previous cardiac history and justify the necessity for ECG monitoring during the acute phase of stroke. The concentration of cardiac-specific troponin (cTn) may be helpful in identifying these disorders. It is a highly sensitive and specific marker of cardiomyocyte damage. Troponin I concentration begins to rise within the first 3 to 8 hours after the onset of chest pain, peaks at 18 to 24 hours and remains elevated for 4 to 7 days after infarction (9).
Studies that have examined the prognostic value of elevated cTn level and its association with a higher risk of death and further vascular events are of interest to cardiologists and other specialists (10-13). These studies emphasize the importance of the cTn concentration measurement to assess mortality risk in patients with internal and/or systemic diseases. The identification of mechanisms contributing to elevated cTnI concentrations in IS patients would enable the identification of patients at especially high risk for cardiac complications. As a result, appropriate treatment would be initiated earlier, resulting in a better prognosis.
AIM
The goals of this study were: to assess the influence of elevated cTnI concentration in patients with IS on the course of stroke and survival prognosis; to characterize the potential relationship between cTnI concentration and other factors influencing the course of stroke, and to identify any correlations between lesion location in the central nervous system (CNS) and elevations in cTnI concentrations following IS.
MATERIAL AND METHODS
This was a hospital-based, prospective, observational, case-control study conducted at the Department of Neurology and Epileptology, Medical Centre of Postgraduate Education, Warsaw, Poland. The study was approved by the Medical Centre of Postgraduate Education Ethics Committee. Two hundred and sixty-one of acute stroke patients were consecutive admitted to the Department between March 2003 and June 2005. Inclusion criteria included: time from stroke onset less than 72 hours, age less than 85 years, stable coronary artery disease (CAD), without history of myocardial infarction (MI) in the four weeks prior to study entry. The study group consisted of 128 women (49%) and 133 men (51%) with IS who were transferred from the Emergency Room directly to the Department. The study group was divided into subgroups according to their cTnI concentration and patient death or survival. The first subgroup consisted of subjects with IS who demonstrated or did not demonstrate elevated cTnI concentrations on admission. Whereas the second subgroup consisted of patients who died or survived throughout the observation period. For each patient, the study period lasted six months starting from the onset of stroke symptoms. At one and six months after IS, the neurological status was re-evaluated or the death of the patient was registered. Patients who failed to show up for follow-up appointments were telephoned at home, and data concerning their health and course of disease were obtained. When it was otherwise impossible to obtain these data, we asked the Warsaw Registrar’s Office for help in identifying patients who died during the study period. Additional tests were carried out in accordance with the European Stroke Initiative guidelines (14). A computed tomographic (CT) imaging study was performed to confirm IS by excluding other reasons for the observed neurological deficits. A standard 12-lead ECG was also recorded routinely on admission. The cTnI concentration was measured once in a blood sample collected on admission, using the AxSYM Troponin-I and AxSYM Troponin-I ADV (starting in October 2004) tests (Abbott Laboratories). Because it was impossible to linearly convert “earlier” cTnI concentrations to “more modern” ones, two ranges were defined with the following nominal values: 0 = cTnI concentrations within the reference range for the healthy population (99th percentile) (earlier test version 0 – 0.3 ng/ml, modern version 0 – 0.04 ng/ml), 1 = cTnI concentrations above reference values (earlier test version > 0.3 ng/ml, modern version > 0.04 ng/ml).
STATISTICAL ANALYSIS
A parametric Students t-test for independent groups and its nonparametric counterpart, the U-test, were used to determine the significance of the differences between variables in the groups analyzed. A chi-square test with Yates’ correction and Fisher’s exact test for counts less than five were employed to demonstrate a relationship between studied variables. A chi-square Mantel-Haenszel test was applied to analyze the mutual influence of given variables in separate subgroups for several independent two-way tables (2 x 2). A Kaplan-Meier method and log-rank test were employed to analyze the survival time. Multivariate logistic regression analysis using Enter and Forward stepwise Wald modeling was applied to determine the influence of selected independent factors on the prevalence of elevated cTnI concentration. A nonparametric Cox proportional hazard model using the Forward stepwise Wald method was employed to determine the influence of analyzed factors on increased prediction of mortality during the first 30 and 180 days after onset of IS symptoms. The results were assumed to be reliable at a significance level α < 0.05. Statistical analyses were performed using the SPSS version 11.5 (SPSS Inc.).
RESULTS
Sixty-seven patients (25.7%) died during the six-month observation period. Early mortality (< 30 days) occurred in 42 cases (16.1%). Elevated cTnI concentration on admission was observed in 16.5% (43/261) of the patients included in the study and in 28.4% (19/67) of the patients who died during the observation period (p < 0.002) (U-test). An elevated cTnI on admission was observed regardless of the length of the survival period i.e. either < 30 days (p < 0.021) or > 30 days after IS (p < 0.002) (U-test). The most common locations for the ischemic lesion were the right (42%, 73/174) and left (33.3%, 58/174) middle cerebral artery (MCA) territories. There was a significant difference between groups with respect to frequency of lesion location in the right MCA territory. This lesion location was observed in 35.1% (46) of patients who survived throughout and 62.8% (26) of patients who died during the study period (p < 0.001) (U-test). The features of cerebral edema in the CT scan taken on admission to hospital in 19.4% (50) patients with IS but in 28.8% (19) of those who died and in 16.1% (31) patients who survived were present (p < 0.025) (U-test). No abnormalities on 12-lead ECG were observed in 27.2% (72) of patients with IS. On ECG, the frequency of abnormalities was significantly different between groups (heart rate [HR] > 100/min and features of previous MI). The prevalence of features of acute MI, HR < 50/min and repolarization abnormalities trended towards significance (U-test) (tab. 1).
Table 1. Prevalence of certain ECG abnormalities in patients with ischemic stroke who survived or died during the observation period.
HR – Heart Rate; AF – Atrial Fibrillation; SVEB – Supraventricular Ectopic Beats; VEB – Ventricular Ectopic Beats; LBBB – Left Bundle- -Branch Block; RBBB – Right Bundle-Branch Block; A-V I° block – Atrioventricular Block first degree
We used logistic regression with the stepwise Wald method to analyze independent factors (age, gender, arterial hypertension, CAD, atrial fibrillation – AF, history of stroke, history of MI, diabetes mellitus, hyperlipidemia, smoking history, alcoholism, severity of neurological status according to Scandinavian Stroke Scale – SSS, elevated inflammation markers, serum glucose level, location of the lesion within the CNS and type of ECG abnormality) and found that the presence of ventricular ectopic beats (VEB) and repolarization abnormalities on ECG, localization of stroke lesion to the right MCA territory, elevated glucose and C-reactive protein (CRP) concentrations on admission were associated with cTnI concentration elevation (tab. 2).
Table 2. Prognostic model of hazard of cTnI increase in ischemic stroke.
VEB – Ventricular Ectopic Beats; MCA – Middle Cerebral Artery; CRP – C-Reactive Protein
A chi-square Mantel-Haenszel test used to analyze the influence of variables on one another in separate subgroups revealed a relationship between early post-stroke mortality in patients with elevated cTnI concentration on admission and presence of HR > 100/min (p < 0.024) or VEB on ECG (p < 0.023). The same relationship applied for late mortality (> 30 days) occurs when of VEB is presented on ECG (p < 0.02).
Similarly, we identified a relationship between early and late mortality following stroke in patients with elevated cTnI concentration on admission and stroke lesion in right MCA territory (p < 0.010 and p < 0.017 for early and late mortality, respectively, via the chi square Mantel-Haenszel test). An elevated cTnI concentration on admission was more frequent only in patients who died within 30 days due to cardiac complications, compared to subjects who died later in the observation period (p < 0.001) (U-test). No such correlation was observed for other causes of death.
A Kaplan-Meier method with significance evaluating log-rank test was used to analyze 30-day survival rates in subjects with elevated cTnI concentrations on admission and indicated that 72% of these patients survived for 30 days vs. 86% of patients with normal cTnI concentrations on admission (fig. 1).
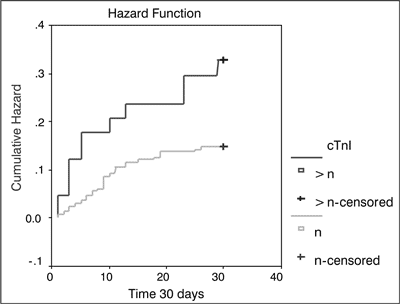 Fig. 1. Death hazard for early mortality after ischemic stroke on dependency cTnI concentration (p < 0.016).
cTnI > n-cTnI concentration above norm; cTnI > n-censored – cTnI concentration above norm for censored events; cTnI n – normal cTnI concentration; cTnI n-censored – normal cTnI concentration for censored events However, analysis of 180-day survival in subjects with elevated cTnI concentrations on admission revealed that 56% of these patients survived this 180-day period vs. 78% of patients with normal cTnI concentrations on admission (fig. 2).
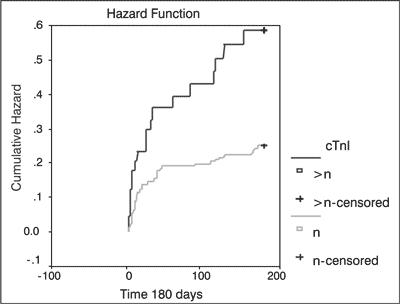 Fig. 2. Death hazard for late mortality after ischemic stroke on dependency cTnI concentration (p < 0.013).
Logistic regression based on a nonparametric Cox proportional hazard model using the Enter method was employed to evaluate the influence of an elevated cTnI concentration on admission as an independent risk factor for mortality and 6 months survival period. We found that patients with elevated cTnI concentrations had 2.21-fold (95% CI 1.14-4.34; p < 0.019) and 2.33-fold (95% CI 1.37-3.96; p < 0.002) higher 30- and 180-day mortality risk, respectively. A stepwise regression analysis based on a nonparametric Cox proportional hazard model using a Forward stepwise Wald method was then employed to determine the influence of independent risk factors on mortality and the survival period. The following independent variables were considered: age, gender, risk factors for cardiovascular diseases, severity of neurological status according to SSS, markers of inflammation (CRP, leukocytosis), cTnI concentration, glucose level, lesion location within the CNS, and type of ECG abnormalities. It was demonstrated that increased leukocytosis on admission, more severe neurological status according to SSS and brain edema on head CT were independent risk factors for 30-day mortality. The 180-day mortality risk from IS was associated with more severe neurological status according to SSS, increased leukocytosis on admission and repolarization abnormalities on ECG (tab. 3).
Table 3. Prognostic model of death hazard < 30 and > 30 days after ischemic stroke.
DISCUSSION
The main thesis of this paper was to determine whether elevated cTnI concentration on admission in patients with IS has any predictive value for unfavorable prognosis, which was defined as death within the first month or six months after onset of stroke symptoms. There are many controversies surrounding this topic. Results similar to those reported herein were presented in an article from Great Britain (15). Using a multivariate logistic regression model to compare known risk factors for death within 30 days after IS. Barber et al. concluded that an elevated cTn concentration is not an independent prognostic factor. Univariate analysis revealed, however, that an elevated cTnI concentration (> 0.2 ng/ml) is an independent risk factor for early mortality, which is increased 3.36-fold (95% CI 1.21-9.26). The latest study on this subject confirmed that elevations of serum cTnT are associated with a worse outcome but the predictive value of elevated cTnT levels is less good than a stroke severity according to National Institute of Health Stroke Scale (NIHSS) (16). On the other hand, Etgen et al. stated that in patients with IS, elevated TnT (> 0.03 ng/ml) and cTnI (> 0.03 ng/ml) levels are not independent risk factors for increased mortality in the three months following IS (17). Despite these observations, the majority of authors emphasize the usefulness of cTn concentration measurement for mortality risk assessment in patients with IS. James et al. (18) were the first to report on this topic. They demonstrated that an elevated cTnT concentration (> 0.1 ng/ml) in IS patients increases in-hospital mortality risk 3.2 times (95% CI 1.7-5.8). Researchers from the Mayo Clinic found that elevated cTn levels (> 0.01 ng/ml) are associated with a 2.4-fold higher risk of death within one year following IS, compared to patients with normal cTn levels (19). An elevated cTnI concentration was found to be an independent risk factor for six-month mortality after IS, with HR = 2.14 (95% CI 1.13-4.05) for concentrations of 0.1-0.39 ng/ml and HR = 2.44 (95% CI 1.21-5.02) for concentrations > 0.4 ng/ml (20). Danish researchers employed a multivariate logistic regression model with dependent variables including death or dependency on other people lasting more than three months after IS and demonstrated that cTnI > 0.1 ng/ml is an independent prognostic factor (OR = 4.1; 95% CI 1.3-14.5) (21), Fure et al. concluded that the prognostic value of a positive troponin test (cTnT > 0.04 ng/ml) in predicting short-term mortality is 0.69 (22). An elevated cTnT concentration (> 0.03 ng/ml) was also an independent prognostic factor for long-term mortality. Jensen et al. designed a multivariate analysis that included age, severity of neurological deficits according to SSS, and heart and/or renal failure and demonstrated that the two-year mortality risk increased 3.39 times (95% CI 1.34-8.60). Univariate analysis showed that the mortality risk in patients with a positive troponin test was HR = 7.92 (95% CI 4.03-15.53) (23). From these studies, we can conclude that an elevated cTn concentration is associated with an increased risk of mortality following IS that is equivalent to the risk posed by acute coronary syndromes (ACS). Kerr et al. estimated that elevated troponin level after acute stroke occurring in up 1 in 5 patients (24).
The results presented herein indicate that stroke within the right MCA territory increases the probability of an elevated cTnI concentration. The MCA supplies the insula, which damaged causes dysregulation of the autonomous nervous system. Some authors have postulated a relationship between an elevation of cTn levels in stroke patients and activation of the sympathetic nervous system, but only indirect evidence for this is available. Barber et al. proved that elevated cTn concentrations in IS patients are associated with elevated epinephrine concentrations (15), whereas Christensen et al. demonstrated that the former is associated with an increased cortisol concentration (21). Only two paper in the available literature has reported a correlation between elevated cTn levels and the insula damage. Ay et al. demonstrated in a group of 50 patients that the stroke territory within the CNS that is associated with elevated cTnT levels represents the right insula and right inferior parietal lobule (25). Song et al. also proved that insular-lobe involvement was independently related to elevated serum cTnT (OR = 2.59, 95% CI 1.89-3.16) but without distinction of side of MCA territory (26). Because coronary vessels are richly innervated with α- and β-adrenergic receptors, this finding supports the idea that the sympathetic system plays a dominant role among the factors that contribute to autoregulation of coronary blood flow (27). Other authors do not imply any such relationship (15, 28).
Repolarization abnormalities and VEB were also found to be independent factors influencing elevated cTnI levels. Studies of this issue have yielded data that are inconsistent, relatively new and not fully explained. In their study of a Danish population, Christensen et al. (29) were not able to demonstrate a relationship between the presence of ECG abnormalities and elevated cTnI concentrations in patients with IS. The Turkish authors Apak et al. reported that ECG abnormalities suggesting MI are significantly more frequently observed in IS patients with elevated cTnT (30) and cTnI (31). An additional conclusion from these studies was the equivalence of the ability of cTnT vs. cTnI to identify cardiomyocyte damage. According to Di Angelantonio et al. a statistical probability of an elevation in cTnI serum concentrations exists in the presence of inverted T-waves and ST-T-segment depression in patients with IS (20). Fure et al. (22) reached similar conclusions and stated that the presence of pathological Q-waves and ST-T-segment depression on the ECG increases the probability of elevated cTnT concentration in patients with IS. The results of Barber et al. (15) are the most similar to ours, as they demonstrated that IS patients with elevated cTnI concentration are more prone to exhibiting ischemic ECG abnormalities. Kerr et al. assessed this relationship on the level OR = 3.03 (95% CI 1.49-6.17) (24).
An elevated CRP concentration on admission is an independent factor underlying an elevated cTnI concentration. Christensen et al. (21) published an article concerning inflammation and the stress response in the acute phase of stroke. They demonstrated that the cTnI concentration correlates significantly with increased leukocytosis and elevated glucose, TNF-α and cortisol concentrations but not with CRP levels. Elevated CRP concentrations were observed more frequently in patients with myocardial damage who simultaneously exhibited elevated cTnI concentrations (32). Such data indicate that the inflammatory process and stress response in the acute phase of stroke may parallel the heart’s reaction because arteriosclerotic plaques develop throughout the entire arterial system. Therefore, it can be concluded that cTn (a marker for cardiomyocyte necrosis) and CRP (an inflammatory marker) complement each other in the risk assessment of cardiac complications in patients with IS. Furthermore, repolarization abnormalities may indicate MI as well.
An elevated glucose level on admission is another independent factor for an increased cTnI concentration. A similar correlation has been observed in patients with ACS (33). The issue of coexistence of elevated cTn and glucose concentrations in acute brain syndromes has not been studied to date. Coagulation changes are responsible for the correlation between increased glucose level and cTn release. Glucose concentration is an independent prognostic factor for platelet-dependent clot formation in patients with coronary disease (34). Increased glycaemia is a well-known phenomenon that occurs in response to the adrenergic stimulation following the release of stress hormones such as cortisol and noradrenaline as well as after damage to the central autonomic control centers (35).
**This study was supported by a grand from the Medical Centre of Postgraduate Education in Warsaw (No 501-2-1-13-54/03). This paper was presented at the World Congress on Controversies in Neurology (CONY, Prague, Czech Republic, October 8-11.2009) and was awarded the Best Poster Prize. Piśmiennictwo
1. Berrior S, Amarenco P: Anomalies electrocardiographiques a la phase aigue de l’infarctus cerebral. Sang Thromb Vaiss 2001; 13: 224-230.
2. Kopelnik A, Zaroff JG: Neurocardiogenic injury in neurovascular disorders. Crit Care Clin 2006; 22: 733-752.
3. Oppenheimer SM: Neurogenic cardiac effects of cerebrovascular disease. Curr Opin Neurol 1994; 7: 20-24.
4. Tatschl C, Stollberger C, Matz K et al.: Insular involvement is associated with QT prolongation: ECG abnormalities in patients with acute stroke. Cerebrovasc Dis 2006; 21: 47-53.
5. Abboud H, Berroir S, Labreuche J et al.: Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol 2006; 59: 691-699.
6. Cheung RT, Hachinski V: The insula and cerebrogenic sudden death. Arch Neurol 2000; 57: 1685-1688.
7. Tokgozoglu SL, Batur MK, Topuoglu MA et al.: Effects of stroke localization on cardiac autonomic balance and sudden death. Stroke 1999; 30: 1307-1311.
8. Sander D, Klingelhofer J: Stroke-associated pathological sympathetic activation related to size infarction and extent of insular damage. Cerebrovasc Dis 1995; 5: 381-385.
9. Bodor GS: Cardiac troponin-I: A highly specific marker for myocardial infarction. J Clin Immunoassay 1994; 17: 40-44.
10. Heidenreich PA, Alloggiamento T, Melsop K et al.: The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol 2001; 38: 478-485.
11. Hudson MP, O’Connor CM, Gattis W et al.: Implications of elevated cardiac troponin T in ambulatory patients with heart failure: a prospective analysis. Am Heart J 2004; 147: 546-552.
12. Ammann P, Maggiorini M, Bertel O et al.: Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol 2003; 41: 2004-2009.
13. Mehta NJ, Khan IA, Gupta V et al.: Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004; 95: 13-17.
14. European Stroke Initiative Executive Committee, EUSI Writing Committee, Olsen TS, Langhorne P, Diener HC et al.: European Stroke Initiative Executive Committee Recommendations for Stroke Management-update 2003. Cerebrovasc Dis 2003; 16: 311-337.
15. Barber M, Morton JJ Macfarlane PW et al.: Elevated troponin levels are associated with sympathoadrenal activation in acute ischaemic stroke. Cerebrovasc Dis 2007; 23: 260-266.
16. Ghali J, Allison D, Kleinig T et al.: Elevated serum concentrations of troponin T in acute stroke: what do they mean? J Clin Neurosci 2010; 17: 69-73.
17. Etgen T, Baum H, Sander K et al.: Cardiac troponins and N-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke 2005; 36: 270-275.
18. James P, Ellis CJ, Whitlock RML et al.: Relation between troponin T concentration and mortality in patients presenting with an acute stroke: observational study. BMJ 2000; 320: 1502-1504.
19. Gilmore RM, Bhagra A, Stead LG: Serum troponin as a predictor of mortality after acute ischemic stroke. Acad Emerg Med 2005; 12 (suppl. 1): 169.
20. Di Angelantonio E, Fiorelli M, Toni D et al.: Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry 2005; 76: 76-81.
21. Christensen H, Johannesen HH, Christensen AF et al.: Serum cardiac troponin I in acute stroke is related to serum cortisol and TNF-alpha. Cerebrovasc Dis 2004; 18: 194-199.
22. Fure B, Bruun Wyller T, Thommessen B: Electrocardiographic and troponin T changes in acute ischaemic stroke. J Intern Med 2006; 259: 592-597.
23. Jensen JK, Kristensen SR, Bak S et al.: Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol 2007; 99: 102-108.
24. Kerr G, Ray G, Wu O et al.: Elevated troponin after stroke: a systematic review. Cerebrovasc Dis 2009; 28: 220-226.
25. Ay H, Koroshetz WJ, Benner T et al.: Neuroanatomic correlates of stroke-related myocardial injury. Neurology 2006; 66: 1325-1329.
26. Song HS, Back JH, Jin D et al.: Cardiac troponin T elevation after stroke: relationships between elevated serum troponin T, stroke location, and prognosis. J Clin Neurol 2008; 4: 75-83.
27. Baumgart D, Heusch G: Neuronal control of coronary blood flow. Basic Res Cardiol 1995; 90: 142-159.
28. Christensen H, Boysen G, Christensen AF: Insular lesions, ECG abnormalities, and outcome in acute stroke. J Neurol Neurosurg Psychiatry 2005; 76: 269-271.
29. Christensen H, Christensen AF, Boysen G: Abnormalities on ECG and telemetry predict stroke outcome at 3 months. J Neurol Sci 2005; 234: 99-103.
30. Apak I, Iltumur K, Tamam Y et al.: Serum cardiac troponin T levels as an indicator of myocardial injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp Med 2005; 205: 93-101.
31. Iltumur K, Yavavli A, Apak I et al.: Elevated plasma N-terminal pro-brain natriuretic peptide levels in acute ischemic stroke. Am Heart J 2006; 151: 1115-1122.
32. Trooyen M, Indredavik B, Rossvoll O et al.: Myocardial injury in acute stroke assessed by troponin I. Tidsskr Nor Laegeforen 2001; 121: 421-415.
33. Kennon S, Barakat K, Hitman GA et al.: Angiotensin-converting enzyme inhibition is associated with reduced troponin release in non-ST-elevation acute coronary syndromes. J Am Coll Cardiol 2001; 38: 724-728.
34. Schechter M, Merz NB, Paul-Labrador MJ et al.: Blood glucose and platelet-dependent thrombosis in patients with coronary artery disease. J Am Coll Cardiol 2000; 35: 300-307.
35. Allport LE, Butcher KS, Baird TA et al.: Insular cortical ischemia is independently associated with acute stress hyperglycemia. Stroke 2004; 35: 1886-1891.
otrzymano/received: 2013-07-17 zaakceptowano/accepted: 2013-09-04 Adres/address: *Urszula Fiszer Department of Neurology and Epileptology Medical Centre of Postgraduate Education ul. Czerniakowska 231, 00-416 Warszawa tel.: +48 (22) 629-43-49; fax: +48 (22) 584-13-06 e-mail: kl.neurologii@szpital-orlowskiego.pl Artykuł Podwyższony poziom troponiny I – czynnik złego rokowania w udarach niedokrwiennych mózgu w Czytelni Medycznej Borgis. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








