|
© Borgis - Postępy Nauk Medycznych 9, s. 634-639
*Tomasz Ociepa, Emilia Sawka, Paulina Panek, Magdalena Wołczyk, Tomasz Urasiński
Ocena przydatności jednoczasowego oznaczania liczby krwinek białych i stężeń białka c-reaktywnego oraz prokalcytoniny w przewidywaniu przebiegu zakażenia u dzieci z ostrą białaczką limfoblastyczną i implantowanym centralnym cewnikiem naczyniowym
The value of concomitant measurement of white blood count, C-reactive protein and procalcitonin in predicting the course of infection in children with acute lymphoblastic leukemia and central venous catheter
Clinic of Pediatric Hematology and Oncology, Pomeranian Medical University, Szczecin
Head of Clinic: prof. Tomasz Urasiński, PhD, MD Streszczenie
Wstęp. Zakażenia odcewnikowe odpowiadają za aż 44% wszystkich epizodów bakteremii u dzieci z ostrą białaczką limfoblastyczną (obl). Wczesna ich identyfikacja oraz odpowiednio intensywne leczenie mogą wpłynąć na poprawę wyników leczenia dziecięcej obl. Cel. Ocena przydatności jednoczasowego oznaczania liczby krwinek białych (WBC), stężeń białka c-reaktywnego (CRP) oraz prokalcytoniny (PCT) w przewidywaniu przebiegu zakażeń u dzieci z obl. Materiał i metody. Analizowano początkową WBC oraz stężenia CRP i PCT w przebiegu 51 epizodów gorączki u 23 dzieci z obl i implantowanym cewnikiem naczyniowym (CVC). U wszystkich pacjentów zastosowano empiryczną antybiotykoterapię. Wyniki. Podwyższone początkowe stężenia PCT i CRP stwierdzono odpowiednio w 22/51 (43,1%) i 43/51 (84,3%) analizowanych przypadkach. Średni czas trwania gorączki, jak również średnie stężenie CRP były istotnie wyższe w przypadkach z początkową WBC poniżej 500/μl. Nie wykazano korelacji pomiędzy stężeniami CRP i PCT, jednakże średnie stężenia CRP jak również liczba dodatnich wyników posiewów krwi były istotnie wyższe w epizodach z podwyższonym początkowym stężeniem PCT. Liczba dodatnich wyników posiewów krwi była również istotnie wyższa w epizodach z jednoczasowym podwyższeniem stężeń CRP i PCT. Stwierdzono wyższe początkowe stężenia PCT ale nie CRP w epizodach z dodatnim wynikiem posiewu krwi. Wnioski. Łączna ocena WBC oraz stężeń CRP i PCT jest przydatna w przewidywaniu przebiegu zakażeń u gorączkujących dzieci z obl i implantowanym centralnym cewnikiem naczyniowym. Umożliwia ona również wyselekcjonowanie pacjentów o wysokim ryzyku rozwoju bakteremii. Słowa kluczowe: białaczka, dzieci, zakażenie, prokalcytonina, białko c-reaktywne
Summary
Introduction. Catheter related infections are responsible for up to 44% of episodes of bacteremia in children with acute lymphoblastic leukemia (ALL). Early prediction and intensive treatment of this complication may improve survival for children with ALL. Aim. To assess the value of concomitant measurement of white blood count (WBC), C-reactive protein (CRP) and procalcitonin (PCT) in predicting the course of infection in children with ALL. Material and methods. 51 febrile episodes recorded in 23 children with ALL and implanted central venous catheter (CVC) were analyzed with respect to initial WBC, CRP, PCT and CVC bacterial culture. Patients were treated with empiric antimicrobial therapy. Results. Elevated values of PCT were found in 22/51 (43.1%) whereas values of CRP were elevated in 43/51 (84.3%) of studied episodes. Mean number of febrile days and mean CRP values were statistically higher in episodes with initial WBC less than 500/μl. There was no correlation between values of CRP and PCT however mean values of CRP and the number of episodes with positive CVC blood culture were statistically higher among those with elevated PCT. The number of positive CVC blood cultures was statistically higher in episodes where both CRP and PCT levels were elevated. There was higher initial concentration of PCT but no CRP in episodes with positive blood cultures. Conclusion. WBC, CRP and PCT, when used together are useful indicators of the infection’s course in febrile patients with ALL and CVC. They also allow to select patients at high risk of bacteremia. Key words: leukemia, children, infection, procalcitonin, c-reactive protein
Introduction
Acute lymphoblastic leukemia (ALL) is the commonest form of childhood cancer. Now, in the advent of 21st century the majority of children with this diagnosis may enjoy long-lasting remission which in most of these patients can be considered as a cure (1). However up to 20% of children with ALL suffer from treatment failures. Most of them are relapses, but 3-15% of all children with ALL die in remission and the direct cause of their death are infections (2-4).
Central venous catheters (CVC) are routinely inserted in children with ALL allowing permanent access to venous circulation for blood sampling as well as drugs administration, blood products transfusing and parenteral nutrition. From the other site the insertion of CVC in a child with ALL is associated with a high risk of bacteremia. The rate of catheter related bacteremia in children with ALL and CVC can be as high as 44% (5, 6). It is extremely dangerous since in most of patients it occurs during profound neutropenia resulting from intensive chemotherapy. Early prediction and vigorous treatment of this complication from the very beginning may result in better outcome for children with ALL.
Two laboratory parameters: C-reacting protein (CRP) and procalcitonin (PCT) may be useful in predicting severity of infection in children with ALL. CRP is a protein produced by the liver and the adipose tissue, which enhances complement binding. It’s concentration is less than 5 mg/l in healthy individuals and rises to maximum values within 24-72 hours in response to inflammation (7). It is believed that PCT is an earlier and more specific marker of infection (8). PCT is 14-kDa prohormone of calcitonin that is synthetized by parafollicular cells of the thyroid as well as the neuroendocrine cells of the lung and the intestine. It has been shown that serum PCT levels raise in response to the presence of bacterial endotoxins in the blood; that is why PCT is considered as the marker of bacteremia in adults as well as in children (9, 10).
Aim
This study was performed in order to assess the value of concomitant measurement of white blood count, C-reactive protein and procalcitonin in predicting the course of infection in children with acute lymphoblastic leukemia and central venous catheter.
Material and methods
This retrospective study, conducted between February 1st 2007 and January 31st 2010 comprised children with ALL and implanted CVC, who developed at least one episode of fever of unknown origin that is body temperature over 38 degrees Celsius measured with the use of infrared forehead thermometer (Microlife), with no other clinical manifestation except of fever and no site of infection seen on physical examination or on imaging (e.g. chest x-ray). There were 51 episodes recorded in 23 children (12 girls and 11 boys) aged 2-14 years (mean 7.06, median 6 years).
In each of these patients blood was sampled routinely for WBC, CRP as well as PCT. PCT was measured with the use of electrochemiluminescence method (ECLIA) for quantitative determination of procalcitonin (Roche), CRP – with the use of immunoturbidimetric method for quantitative determination of C-reactive protein (Roche). Values of CRP below 5 mg/l and PCT below 0.5 ng/ml were considered normal. A sample of peripheral blood was also drawn from the catheter for bacterial cultures. These were followed by introduction of empiric antimicrobial therapy consisting of piperacillin/tazobactam (112.5 mg/kg every 8 hours) alone or in combination with amikacin (15 mg/kg once daily); the decision regarding a form of treatment was made by a physician in charge.
In patients with no clinical response to the first line antimicrobial therapy i.e. with no resolution of fever within 48 h treatment was modified: either according to the result of CVC bacterial culture or – if culture was negative – to combination of meropenem (20 mg/kg 3 times daily) plus vancomycin (20 mg/kg 3 times daily), than after subsequent 48 h – as a third step – an antifungal agent (amphotericin B or voriconazole) was added.
Duration of fever was reflected by number of days with at least one spike of body temperature over 38 degrees Celsius measured as mentioned above.
WBC, CRP and PCT values were analyzed in relation to results of central catheter blood culture (positive vs negative) and to duration of fever. The correlation between CRP and PCT values was also studied.
The normality of data was assessed with the use of Kolmogorov-Smirnov test. Statistical analysis of data was performed with the use of Fisher test, Mann-Whitney test, t-Student test and linear correlation model. Values of p < 0.05 were considered statistically significant.
Results
There were no treatment failures recorded in the group studied; in all fever episodes antimicrobial therapy resulted in resolution of fever. Mean number of febrile days as well as mean CRP values were statistically higher in episodes with initial WBC less than 500/μl (5.20 vs 10.73 days; p < 0.03, and 37.2 mg/l vs 106.8 mg/l; p < 0.005) respectively however PCT values did not differ significantly. These data are presented in table 1.
Table 1. Mean duration of fever, values of CRP and PCT in episodes with WBC ≥ 500/μl and < 500/μl.
Elevated values of PCT were found in 22/51 (43.1%) whereas values of CRP were elevated in 43/51 (84.3%) of studied episodes.
There was no correlation between values of CRP and PCT (r = 0.4983) however mean values of CRP (34.82 vs 87.78; p < 0.01) and the number of episodes with positive CVC blood culture (8/22 vs 1/29; p < 0.005) were statistically higher among those with elevated PCT. These data are presented in figure 1 and table 2.
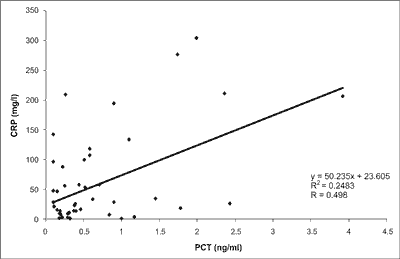 Fig. 1. Correlation between CRP and PCT in febrile children with ALL and implanted CVC.
Table 2. Mean duration of fever, values of CRP and number of positive CVC blood culture in episodes with normal and elevated PCT concentration.
Moreover episodes with normal vs elevated CRP concentration did not differ significantly with respect to duration of fever (4.12 vs 7.72; p = 0.28), mean concentration of PCT (0.52 vs 0.69 ng/ml; p = 0.59) and number of positive CVC blood culture results (0/8 vs 9/43; p = 0.32). These data are presented in table 3.
Table 3. Mean duration of fever, mean values of PCT and number of positive CVC blood culture in episodes with normal and elevated CRP concentration.
It has been also shown that the number of positive CVC blood cultures was statistically higher in episodes where both studied parameters i.e. CRP and PCT were elevated. These data are presented in table 4.
Table 4. Number of positive CVC blood culture in episodes with elevated both PCT and CRP concentration.
There was higher initial concentration of PCT but no CRP in episodes with positive blood cultures. These data are presented in table 5.
Table 5. Mean duration of fever, values of CRP, PCT and number of episodes with initially elevated PCT concentration in episodes with positive and negative CVC blood cultures.
Discussion
The role of PCT and CRP as markers of infection and predictors of its severity has been extensively studied by several authors. Van Rossum et al. reported on the utility of PCT in early identification of bacterial infections in infants and children. He stated that initial concentration of PCT is a good predictor of severe sepsis development in children (10). This observation was also supported by data published by Fioretto et al. (11). Moreover the same group of Fioretto as well as the group of Casado-Flores published data indicating that serum PCT level was superior to serum CRP level in predicting sepsis and septic shock in children (12, 13). Finally Martinez-Albarran et al. analyzed course of infections in 54 consecutive neutropenic children with cancer treated in a single center and their data seem to confirm the usefulness of PCT estimation (4). There is a debate regarding the cut-off value for PCT. The study of Lodahl et al. of 230 febrile episodes in 85 children indicated that PCT with cut off value of > 0.4 ng/ml is more accurate than CRP in determining the severity of infection (14). Moreover Endo et al. reported the usefulness of PCT cut-off level of 2 ng/ml in differentiating sepsis and severe sepsis (15).
To our best knowledge this is the first report analyzing the usefulness of serum PCT and CRP concentration in predicting severity of infection in febrile children with ALL and implanted CVC. Most infections in children with ALL are treated according to so called fever-driven protocols. It means that the antimicrobial therapy is initiated when fever occurs and is subsequently modified within 48-72 hours if fever persists. It underlines the need for markers which at the beginning of a febrile episode would predict its severity thus forming a rational basis for selection of antibiotics.
In the presented study elevated concentration of CRP was found in 43 out of 51 studied episodes of infection (84.3%) whereas values of PCT were initially elevated in only 22 out of 51 episodes (43.1%). It seems to indicate that CRP concentration is an earlier marker of infection in febrile children with ALL. This is in opposition to the opinion presented by Gendrel and Bohuon who stated that PCT is the earliest marker of a bacterial infection (9).
It is of crucial importance to find patients with a bloodstream infection since in neutropenia it may rapidly turn into sepsis and septic shock and the mortality in sepsis reaches 16%, but in severe sepsis and septic shock can be as high as 50 and 80% respectively (16, 17). Interestingly in the group studied the number of episodes with a positive result of CVC blood culture was statistically higher in patients with elevated PCT. This was even more pronounced when elevated PCT was accompanied by elevated CRP (8 of nine positive CVC blood cultures were seen in this subset of patients) even though elevated CRP alone did not predict bacteriemia. Bacterial endotoxins are the most powerful biological substances initiating PCT synthesis (18, 19). It has been found by Kasem et al. that procalcitonin was a rapid marker of bacteriemia in 62 children with fever and a central venous catheter admitted to emergency department (20). The rate of catheter related bacteriemia in children with ALL and CVC can be as high as 44% (5). Our observation on a role of elevated PCT and CRP in predicting bacteriemia in febrile children with ALL and CVC – if confirmed in other studies – may be of clinical significance.
The severity of infection is somehow related to the duration of fever. In our study neither serum PCT nor serum CRP correlated with the number of febrile days. However the duration of fever was significantly longer in patients with severe neutropenia that is with WBC less than 500/μl. It is not surprising since neutropenia is one of the most important factors responsible for increased risk of infections in children with cancer and in severe neutropenia some longer time is needed for hematological reconstruction (21). It is also of interest that in our study patients with low WBC had statistically higher serum CPR levels even though levels of PCT did not differ significantly. This observation seems to indicate that in children with ALL, CVC and severe neutropenia, CRP is a more sensitive marker of infection than PCT.
There is no doubt that children with ALL, implanted CVC and fever must be treated in a hospital setting and an empirical antimicrobial treatment should be initiated as early as possible since any delay in antibiotic initiation can impair the prognosis (16). However selection of antimicrobial agents is still a matter of a debate. Several reports focused on this issue suggest administration of penicillins with beta-lactamase inhibitors, cephalosporins as well as carbapenems and glycopetides as the first line empirical management (22-25). We believe that data presented here will also contribute to creation of more effective management protocols for febrile episodes in children with ALL and CVC.
Conclusions
We conclude that three parameters: WBC, CRP and PCT concentration, when used together are useful indicators of the infection’s course in febrile patients with ALL and CVC:
1. Elevated serum CRP is simply a marker of infection whereas WBC < 500/ml predicts the longer duration of fever.
2. Elevated serum PCT, especially when accompanied by elevated serum CRP is associated with a very high risk of bacteriemia thus indicating the need of vigorous antimicrobial therapy. Piśmiennictwo
1. Pui CH, Campana D, Pei D et al.: Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 2009 Jun 25; 360(26): 2730-2741.
2. Christensen MS, Heyman M, Möttönen M et al.: Nordic Society of Paediatric Haematology and Oncology (NOPHO). Treatment--related death in childhood acute lymphoblastic leukaemia in the Nordic countries: 1992-2001. Br J Haematol 2005; 131(1): 50-58.
3. Lund B, Åsberg A, Heyman M et al.: Nordic Society of Paediatric Haematology and Oncology. Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer 2011 Apr; 56(4): 551-559.
4. Martinez-Albarran M, Perez-Molina Jde J, Gallegos-Castorena S et al.: Procalcitonin and C-reactive protein serum levels as markers of infection in a pediatric population with febrile neutropenia and cancer. Pediatr Hematol Oncol 2009; 26(6): 414-425.
5. Katsibardi K, Papadakis V, Charisiadou A et al.: Blood stream infections throught the entire course of acute lymphoblastic leukemia treatment. Neoplasma 2011; 58(4): 326-330.
6. Fratino G, Molinari AC, Parodi S et al.: Central venous catheter-related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol 2005 Apr; 16(4): 648-654.
7. Du Clos TW: Function of C-reactive protein. Ann Med 2000 May; 32(4): 274-278.
8. Andreola B, Bressan S, Callegaro S et al.: Procalcitonin and C-reactive protein as diagnostic markers of severe bacterial infections in febrile infants and children in the emergency department. Pediatr Infect Dis J 2007; 26(8): 672-677.
9. Gendrel D, Bohuon C: Procalcitonin as a marker of bacterial infection. Pediatr Infect Dis J 2000 Aug; 19(8): 679-687.
10. van Rossum AM, Wulkan RW, Oudesluys-Murphy AM: Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis 2004; 4(10): 620-630.
11. Fioretto JR, Martin JG, Kurokawa CS et al.: Interleukin-6 and procalcitonin in children with sepsis and septic shock. Cytokine 2008; 43(2): 160-164.
12. Fioretto JR, Martin JG, Kurokawa CS et al.: Comparison between procalcitonin and C-reactive protein for early diagnosis of children with sepsis or septic shock. Inflamm Res 2010; 59(8): 581-586.
13. Casado-Flores J, Blanco-Quirós A, Asensio J et al.: Serum procalcitonin in children with suspected sepsis: a comparison with C-reactive protein and neutrophil count. Pediatr Crit Care Med 2003; 4(2): 190-195.
14. Lodahl D, Schrøder H: Procalcitonin adds to diagnosis, but does not reduce initial antibiotics in febrile neutropenic children. Dan Med Bull 2011; 58(3): A4233.
15. Endo S, Aikawa N, Fujishima S et al.: Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother 2008 Jun; 14(3): 244-249.
16. Dellinger RP, Levy MM, Rhodes A et al.: Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41(2): 580-637.
17. Rangel-Frausto MS, Pittet D, Costigan M et al.: The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 1995 Jan 11; 273(2): 117-123.
18. Maruna P, Nedelníková K, Gürlich R: Physiology and genetics of procalcitonin. Physiol Res 2000; 49 (suppl. 1): 57-61.
19. Dahaba AA, Metzler H: Procalcitonin’s role in the sepsis cascade. Is procalcitonin a sepsis marker or mediator? Minerva Anestesiol 2009; 75(7-8): 447-452.
20. Kasem AJ, Bulloch B, Henry M et al.: Procalcitonin as a marker of bacteremia in children with fever and a central venous catheter presenting to the emergency department. Pediatr Emerg Care 2012; 28: 1017-1021.
21. Afzal S, Ethier MC, Dupuis LL et al.: Risk factors for infection-related outcomes during induction therapy for childhood acute lymphoblastic leukemia. Pediatr Infect Dis J 2009; 28(12): 1064-1068.
22. Yilmaz S, Oren H, Demircio?lu F, Irken G: Assessment of febrile neutropenia episodes in children with acute leukemia treated with BFM protocols. Pediatr Hematol Oncol 2008; 25(3): 195-204.
23. Karaman S, Vural S, Yildirmak Y et al.: Comparison of piperacillin/tazobactam and cefoperazone sulbactam monotherapy in treatment of febrile neutropenia. Pediatr Blood Cancer 2011 Jun 14. doi: 10.1002/pbc.23245. [Epub ahead of print] PubMed PMID: 21674768.
24. Zengin E, Sarper N, K?l?ç SC: Piperacillin/tazobactam monotherapy versus piperacillin/tazobactam plus amikacin as initial empirical therapy for febrile neutropenia in children with acute leukemia. Pediatr Hematol Oncol 2011 May; 28(4): 311-320.
25. Erbey F, Bayram I, Yilmaz S, Tanyeli A: Meropenem monotherapy as an empirical treatment of febrile neutropenia in childhood cancer patients. Asian Pac J Cancer Prev 2010; 11(1): 123-126.
otrzymano/received: 2013-07-22 zaakceptowano/accepted: 2013-08-26 Adres/address: *Tomasz Ociepa Clinic of Pediatric Hematology and Oncology Pomeranian Medical University ul. Unii Lubelskiej 1, 71-252 Szczecin tel.: +48 (91) 425-31-60; +48 (91) 425-31-39 e-mail: tociepa@sci.pum.edu.pl |
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








