|
© Borgis - Postępy Nauk Medycznych 9, s. 604-608
*Jan Styczyński1,2, Robert Dębski1,2, Anna Krenska1,2, Krzysztof Czyżewski1,2, Ninela Irga3, Magdalena Szalewska3, Elżbieta Adamkiewicz-Drożyńska3, Marcin Płonowski4, Elżbieta Leszczyńska4, Maryna Krawczuk-Rybak4, Tomasz Ociepa5, Tomasz Urasiński5, Monika Pogorzała1,2, Mariusz Wysocki1,2
Nawroty choroby nowotworowej i powikłania infekcyjne jako najczęstsze przyczyny niepowodzeń po przeszczepieniu komórek krwiotwórczych
Disease relapse and infectious complications as the main cause of stem cell transplant failure
1Department of Pediatric Hematology and Oncology, Collegium Medicum, Bydgoszcz, Nicolaus Copernicus University
Head of the Department: prof. Mariusz Wysocki, MD, PhD 2Jurasz University Hospital, Bydgoszcz Director: Jacek Kryś, M.Sc. 3Department of Pediatric Hematology, Oncology and Endocrinology, Medical University, Gdańsk Head of the Department: prof. Elżbieta Adamkiewicz-Drożyńska, MD, PhD 4Department of Pediatric Oncology and Hematology, Medical University, Białystok Head of the Department: prof. Maryna Krawczuk-Rybak, MD, PhD 5Department of Pediatric Hematology and Oncology, Pomeranian Medical University, Szczecin Head of the Department: prof. Tomasz Urasiński, MD, PhD Streszczenie
Wstęp. Przeszczepienie hematopoetycznych komórek macierzystych (HSCT) jest ważną metodą terapeutyczną w hematologii, onkologii i immunologii. Cel. Celem pracy była analiza wyników HSCT w ośrodku bydgoskim z uwzględnieniem rodzaju dawcy. Materiał i metody. Od roku 2003 do czerwca 2013 w Klinice Pediatrii, Hematologii i Onkologii w Collegium Medicum w Bydgoszczy wykonano 241 zabiegów HSCT, w tym 130 allogenicznych (53 zgodnych rodzinnych i 77 od dawców alternatywnych) oraz 111 autologiczne. U 20 pacjentów wykonano więcej niż jedną transplantację. Wyniki. Ogółem 143/221 (64,7%) pacjentów żyło w dniu analizy. Spośród pacjentów po auto-HSCT, 29/102 (28,5%) zmarło z powodu nawrotu/progresji (n = 25, tj. 24,6%) lub z powikłań poprzeszczepowych (n = 4, tj. 3,9%), w tym toksyczności (n = 3) lub powikłań infekcyjnych (n = 1). Spośród pacjentów po allo-HSCT, 49/119 (41,2%) zmarło z powodu nawrotu/progresji (n = 19, tj. 15,8%) lub z powikłań poprzeszczepowych (n = 30, tj. 25%), w tym powikłań infekcyjnych (n = 18, tj. 60%), GVHD (n = 7, tj. 23,3%) lub toksyczności (n = 5, tj. 16,7%). Średnie przeżycie po HSCT, wyznaczone metodą Kaplana-Meiera, wyniosło 5,8 lat. Całkowite prawdopodobieństwo przeżycia (pOS) wyniosło 0,61 ± 0,03. pOS po allo-HSCT wynosi 0,56 ± 0,06, a po auto-HSCT 0,65 ± 0,06. pOS po HSCT od zgodnego dawcy rodzinnego wynosi 0,66 ± 0,07, a od dawcy alternatywnego 0,49 ± 0,09. Wśród HSCT od dawców niespokrewnionych pOS wyniosło 0,49 ± 0,09 po przeszczepach od dawców zgodnych 10/10 w HLA (MUD-HSCT), 0,52 ± 0,11 po przeszczepach od dawców zgodnych 9/10 w HLA oraz 0,20 ± 0,18 od dawców częściowo zgodnych 8/10 w HLA (MMUD-HSCT). Wnioski. Nawroty choroby nowotworowej i powikłania infekcyjne są najczęstszymi przyczynami niepowodzeń po przeszczepieniu allogenicznych komórek krwiotwórczych, natomiast nawroty choroby nowotworowej są najczęstszymi przyczynami niepowodzeń po przeszczepieniach autologicznych. Słowa kluczowe: terapia wysokodawkowa, komórki hematopoetyczne, przeszczepianie komórek macierzystych, dzieci
Summary
Introduction. Hematopoietic stem cell transplantation (HSCT) is an important therapeutic method in hematology, oncology and immunology. Aim. The general overview of the results of HSCT in Bydgoszcz Transplant Center with respect to donor type. Material and methods. Between 2003 to June 2013, a total number of 241 of hematopoietic stem cell transplantations were performed in Department of Pediatric Hematology and Oncology, Collegium Medicum, Bydgoszcz, including 130 allogeneic (51 family and 60 unrelated donors) and 111 autologous HSCT. In 20 patients, more than 1 transplantation was performed. Results. A total of 143/221 (64.7%) patients were alive at the end of the follow-up. Among auto-HSCT patients 29/102 (28.5%) has died due to disease relapse/progression (n = 25, i.e. 24.6%) or transplant-related complications (n = 4, i.e. 3.9%). TRM included transplant toxicity (n = 3) or infections (n = 1). Among allo-HSCT patients 49/119 (41.2%) has died due to disease relapse/progression (n = 19, i.e. 15.8%) or transplant-related complications (n = 30, i.e. 25%). TRM included infections (n = 18, i.e. 60%), GVHD (n = 7, i.e. 23.3%) or transplant toxicity (n = 5, i.e. 16.7%). The median follow-up of survivors estimated by Kaplan-Meier method was 5.8 years. Probability of overall survival (pOS) for all patients was 0.61 ± 0.03. pOS after allo-HSCT was 0.56 ± 0.06, and after auto-HSCT was 0.65 ± 0.06. pOS after matched family donor HSCT was 0.66 ± 0.07 and after alternative donor HSCT 0.48 ± 0.07. With respect to unrelated donors, pOS was 0.49 ± 0.09 for 10/10 HLA-matched donors (MUD-HSCT), 0.52 ± 0.11 for 9/10 HLA-matched donors and 0.20 ± 0.18 for 8/10 HLA-matched donors. Conclusion. Disease relapse and infectious complications are the main cause of allogeneic stem cell transplant failure, while disease relapse is the main cause of autologous transplant failure. Key words: high-dose therapy, hematopoietic stem cells, stem cell transplantation, children
Introduction
Hematopoietic stem cell transplantations (HSCT) has become an established method as a treatment for refractory blood diseases, with the aim of achieving long-term survival. According to a report from Poltransplant (1), the total number of allogeneic transplantations performed in Poland in 2012 had reached 449, including 263 from unrelated donors (UD) and 186 from matched family donors (MFD), while the number of autologous-HSCT reached 797 in this year. Department of Pediatric Hematology and Oncology in Bydgoszcz has begun HSCT as MFD-HSCT in 2003, and then auto-HSCT from 2004 and UD-HSCT from 2007 (2, 3).
Aim
The objective of the study was the analysis of results of autologous and allogeneic hematopoietic stem cell transplantations and analysis of factors contributing to transplant failure in 10-years experience of single pediatric center.
Material and methods
Patients
All consecutive patients undergoing HSCT in the Department of Pediatric Hematology and Oncology between 2003 and June 2013 were included into the study. The total number of transplants was 241, including 111 auto-HSCT and 130 allo-HSCT. Among allogeneic transplants, 53 were done from MFD and 77 from alternative donor (including 3 haploidentical donor transplants). The recipients of transplants were males in 150 (62%) transplants and females in 91 (38%) cases. The median age of transplant recipients was 11.9 years (range 0.3-32 years). The source of hematopoietic stem cells was peripheral blood (PB) in 173 patients (71.8%), bone marrow (BM) in 65 patients (27.0%) and cord blood (CB) in 3 patients (1.2%). The median follow-up was 1,7 years (range: 0.1-9.3 years). The primary diagnosis were: acute lymphoblastic leukemia (ALL, n = 51), acute myeloid leukemia (AML, n = 48), chronic myeloid leukemia (CML, n = 6), Hodgkin disease (HD, n = 23), non-Hodgkin lymphoma (NHL, n = 16), neuroblastoma (NBL, n = 31), bone marrow failure (BMF/SAA, n = 21), brain tumor (OUN, n = 11), Ewing tumor (EWING, n = 13), Wilms tumor (WT, n = 5), germinal tumors (GT, n = 5), soft tissue sarcomas (STS, n = 3), and primary immunodeficiency syndromes (PID, n = 3). In 145 (60.2%) cases transplant was performed in first remission, in 76 (31.5%) cases in second or incomplete remission, and in 20 (8.3%) cases in more advanced disease. Eighteen patients had multiple transplants, including 16 patients who had 2 transplants and 2 patients who underwent 3 transplants, thus the total number of patients was 221.
Transplant procedures
Patients underwent HSCT according to procedures described previously (3-6). Standard infectious prophylaxis were used (3-6). For GVHD prophylaxis, cyclosporine ± methotrexate were mainly used after transplants PB or BM, and cyclosporine + steroids after cord blood transplants.
End points
Overall survival (OS) was set as the primary end point. OS was defined as time from transplantation to death or last follow-up. Disease free survival (DFS) was defined as time from the transplantation to disease relapse, death during remission or last follow-up. Non-relapse mortality (NRM) was defined as a death not related to disease. Neutrophil recovery was defined as an absolute neutrophil count of at least 0.5 G/L for three consecutive days. Platelet recovery was defined as a count of at least 20 G/L without transfusion support for 7 days. Acute GVHD (aGVHD) was defined in accordance with standard criteria. Chronic GVHD (cGVHD) was evaluated in patients surviving for more than 100 days after allo-HCT and was classified into limited or extensive type.
Statistical analysis
Mean survival was determined by Kaplan-Meier method, with 95% confidence interval (CI). Unadjusted probability of overall survival (pOS), probability of DFS (pDFS) and probability of relapse (pR) were estimated using the Kaplan-Meier method and compared using the log-rank tests. Risk factor analysis was performed using the Cox model. All p-values were 2-tailed and considered statistically significant if the values were less than 0.05. All statistical analyses were performed using the SPSS21 software (SPSS Inc, Chicago, IL, USA).
Results
Engraftment
Neutrophil engraftment was achieved in 232 (96.2%) of 241 transplants. The median time to neutrophil recovery was 14 days (range, 7-34). In a total of 20 patients, a platelet count > 20 G/L was not reached. In the patients that achieved platelet counts of > 20 G/L, the median time to platelet engraftment was 14 days (range, 1-187). The cumulative probabilities of neutrophil and platelet recovery were 96.2 and 91.7%, respectively.
GVHD
In allo-HSCT patients, the cumulative probabilities of aGVHD and cGVHD were 23.8 and 23.6%, respectively. Nineteen (14.6%) patients developed aGVHD grade II or higher, including 8 (6.1%) with grade III or IV, while 15/106 (14.2%) evaluable patients developed extensive cGVHD.
Mortality
A total of 143/221 (64.7%) patients were alive at the end of the follow-up. Among auto-HSCT patients 73/102 (71.5%) were alive, while 29 has died due to disease relapse/progression (n = 25, i.e. 24.5%) or transplant-related complications (n = 4, i.e. 3.8%). TRM included transplant toxicity (n = 3) or infections (n = 1). Among allo-HSCT patients 70/119 (58.8%) were alive, while 49 has died due to disease relapse/progression (n = 19, i.e. 15.8%) or transplant-related complications (n = 30, i.e. 25%). TRM included infections (n = 18, i.e. 60%), GVHD (n = 7, i.e. 23.3%) or transplant toxicity (n = 5, i.e. 4.2%). Overall, 30-days survival was 98 ± 1.4% after auto-HSCT and 95.8 ± 1.4% after allo-HSCT; 100 days survival was 95 ± 2.2% after auto- -HSCT and 80.4 ± 3.7% after allo-HSCT; and 1-year survival was 80.9 ± 4.1% and 64.2 ± 4.5% after auto- -HSCT and allo-HSCT, respectively.
Probability of overall survival
The median follow-up of survivors estimated by Kaplan-Meier method was 5.8 years (95% CI = 5.2-6.5 years). Probability of overall survival (pOS) after all transplants was 0.61 ± 0.03 and it varied with respect to initial diagnosis (tab. 1). pOS after allo-HSCT was 0.56 ± 0.06, and after auto-HSCT was 0.65 ± 0.06 (p = 0.012) (fig. 1). pOS after matched family donor HSCT was 0.66 ± 0.07 and after alternative donor HSCT 0.48 ± 0.07 (p = 0.045). With respect to alternative donors, pOS was 0.49 ± 0.09 for 10/10 HLA-matched donors (MUD-HSCT), 0.52 ± 0.11 for 9/10 HLA-matched donors and 0.20 ± 0.18 for 8/10 HLA-matched donors (p = 0.381). For patients undergoing haploidentical HSCT, pOS = 0.66 ± 0.27.
Table 1. Results of HSCT with respect to diagnosis.
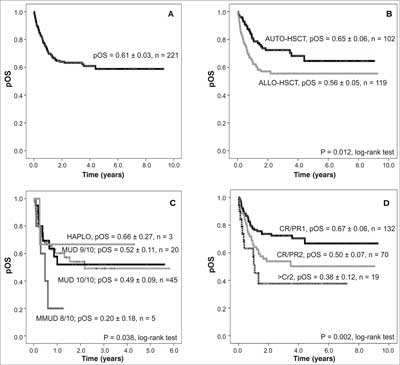 Fig. 1. Results of hematopoietic stem cell transplantation. Probability of overall survival (pOS) after hematopoietic stem cell transplantation: (A) for all patients; (B) after auto-HSCT and after allo-HSCT; (C) after matched unrelated donor HSCT and after mismatched unrelated donor HSCT; (D) with respect to phase of the disease.
HAPLO – haploidentical; (M)MUD – (mis)matched unrelated donor; CR – complete remission; PR – partial remission Discussion
There are 18 hematopoietic stem cell transplant centers in Poland. In 2012, allogeneic transplants from unrelated donors were being performed in 13 of them (1). Our unit has started work, with matched sibling donor HSCT in 2003.
With the number of 33-38 transplants performed each year from 2009, our center has become the second largest pediatric transplant center in Poland, and only in Wrocław the number of pediatric transplants is higher. Obviously, the transplant number is much higher in hematology center for adult patients. Nevertheless, there are more autologous than allogeneic transplants performed for adults. The report from Poltransplant indicated however that Bydgoszcz transplant unit is among leaders performing transplants from unrelated donors (1). Only four centers (Katowice, Wrocław, Poznań and Warszawa) had more unrelated donors transplants done in their large centers (1). Each of these centers has the number of transplants beds at least twice as much as in our center.
Nowadays, all types of hematopoietic stem cell transplantations are being performed in our department: autologous and allogeneic, from matched family donors and from haploidentical donors, from matched and mismatched unrelated donors. All available stem cell sources were used for transplants in our center: peripheral blood, bone marrow and cord blood, both from national and international registries.
Current results of HSCT are mainly dependent on primary diagnosis, clinical stage or grade and previous therapy. The best results were obtained for patients with non-Hodgkin lymphoma, germinal tumors and non-malignant diseases, the worst for patients with central nervous tumors and soft tissue sarcomas. Therapy failure was related to relapse, both after autologous and allogeneic transplants and infections complicating graft-versus-host disease after allogeneic transplants, especially in patients with leukemia. Nevertheless, presented results are comparable with those obtained by a large variety of European transplant centers, cooperating within EBMT (European Group for Blood and Marrow Transplantation). Current improvement in HSCT outcome is dependent both on very good donor match and multidirectional and interdisciplinary supportive care.
The Bydgoszcz center is well recognized in Europe (6-9). Two large international studies are coordinated in out department: study on complications in pediatric donors and therapy of post-transplant lymphoproliferative disorder (7-8). New transplant and supportive care procedures have been introduced in the department (new grading of chronic GVHD, intravenous busulfan, keratinocyte growth factor use). The center actively participates in EBMT, as well as in Pediatric Diseases, Infectious Diseases, and Aplastic Anemia Working Parties, in international project ECIL and cooperates with Columbia University in New York (6-10).
CONCLUSIONS
In summary, our department is the second largest pediatric transplant center in Poland. The results of HSCT obtained in the center are comparable with those of other European transplant centers. Application of transplants from unrelated donors in our center in 2007 has enlarged therapeutic possibilities for our patients. No differences is seen in outcome after matched unrelated donor (10/10 match HLA) and unrelated donor with one HLA disparity (9/10 match HLA) hematopoietic stem cell transplantations. Disease relapse and infectious complications are the main cause of allogeneic stem cell transplant failure, while disease relapse is the main cause of autologous transplant failure.
Acknowledgements
We thank the nursing staff (chaired by Ewa Dembna) of the Pediatric BMT Team for their outstanding support and care of our patients, and physicians from the Department of Pediatric Hematology and Oncology at the Jurasz University Hospital of Bydgoszcz for their continuous support for transplant center. Piśmiennictwo
1. Łęczycka A: Rejestr przeszczepień komórek krwiotwórczych szpiku i krwi obwodowej oraz krwi pępowinowej. Poltransplant. Biuletyn informacyjny. Warszawa 2013; 21(1): 46-51.
2. Styczyński J: Pięć lat Oddziału Transplantacji Szpiku Kostnego w Bydgoszczy: historia, rozwój i perspektywy. Med Biol Sci 2008; 22(3): 67-68.
3. Styczyński J, Dębski R, Krenska A et al.: Transplantacje komórek hematopoetycznych w świetle 5-letnich doświadczeń. Med Biol Sci 2008; 22(3): 157-163.
4. Gmerek A, Styczyński J, Krenska A et al.: Pneumonia in hematopoietic stem cell recipients during early post-transplant period. Med Biol Sci 2009; 23(3): 39-43.
5. Styczyński J, Gil L: Prevention of infectious complications in pediatric HSCT. Bone Marrow Transplant 2008; 42 (suppl. 2): 77-81.
6. Styczyński J, Reusser P, Einsele H et al.: Management of herpes simplex, varicella-zoster and Epstein-Barr virus infection in patients with hematological malignancies and after stem cell transplantation. Guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009; 43: 757-770.
7. Styczyński J, Gil L, Tridello G et al.: Response to rituximab-based therapy and risk factor analysis in EBV-related lymphoproliferative disorders after hematopoietic stem cell transplantation in children and adults: a study from the European Group for Blood and Marrow Transplantation. Clin Infect Dis 2013, in press.
8. Styczyński J, Balduzzi A, Gil L et al.: Risk of complications during hematopoietic stem cell collection in pediatric sibling donors: a prospective European Group for Blood and Marrow Transplantation Pediatric Diseases Working Party study. Blood 2012; 119: 2935-2942.
9. Styczyński J, Einsele H, Gil L, Ljungman P: Outcome of treatment of Epstein-Barr-virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Inf Dis 2009; 11: 383-392.
10. Styczyński J, Tallamy B, Waxman I et al.: A pilot study of reduced toxicity conditioning with busulfan, fludarabine and alemtuzumab prior to allogeneic hematopoietic stem cell transplantation in children and adolescents. Bone Marrow Transplant 2011; 46(6): 790-799.
otrzymano/received: 2013-07-22 zaakceptowano/accepted: 2013-08-26 Adres/address: *Jan Styczyński Katedra Pediatrii, Hematologii i Onkologii Collegium Medicum UMK ul. Curie-Skłodowskiej 9, 85-094 Bydgoszcz tel.: +48 (52) 585-48-60, fax: +48 (52) 585-48-67 e-mail: jstyczynski@cm.umk.pl Artykuł Nawroty choroby nowotworowej i powikłania infekcyjne jako najczęstsze przyczyny niepowodzeń po przeszczepieniu komórek krwiotwórczych w Czytelni Medycznej Borgis. |
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








