|
© Borgis - Postępy Nauk Medycznych 7, s. 501-505
*Joanna Bienias, Anna Mazur, Katarzyna Błaszczyńska, Dorota Król, Stanisław Dyląg
Ocena krwinek płytkowych po procesie kriokonserwacji
Platelets estimation after cryopreservation
Regional Center of Blood Donation and Treatment, Katowice
Head of Regional Center: Stanisław Dyląg, Pharm D, PhD Streszczenie
Wstęp. Koncentraty krwinek płytkowych stosuje się w leczeniu krwawień u pacjentów z małopłytkowością, w celu zapobiegania spontanicznym krwotokom oraz w czasie inwazyjnych zabiegów chirurgicznych. Metodą pozwalającą na dłuższe przechowywanie koncentratów jest kriokonserwacja. Cel pracy. Celem pracy było sprawdzenie, czy metoda otrzymywania koncentratów krwinek płytkowych wpływa na liczbę odzyskanych płytek krwi po procesie kriokonserwacji. Materiał i metody. Analizy prowadzono od czerwca 2006 do stycznia 2010 roku i objęły one 160 koncentratów krwinek płytkowych wcześniej wykonanych metodą aferezy i przez zlewanie kożuszków leukocytarno-płytkowych. Płytki krwi zamrażano, stosując jako środek krioochronny 5% dwumetylosulfotlenek (DMSO) i przechowywano w temperaturze -80°C. Oceniano liczbę krwinek płytkowych przed zamrożeniem i po rozmrożeniu przy użyciu licznika hematologicznego. Wyniki. Średni odzysk rozmrożonych krwinek wyniósł 73,57% i w zależności od zastosowanej metody otrzymywania wahał się od 72,77 do 74,42%. Po przeprowadzonej analizie nie stwierdzono różnic znamiennych statystycznie pomiędzy wynikami odzysku krwinek płytkowych po rozmrożeniu otrzymanych różnymi metodami. Uzyskane średnie wartości dla wszystkich trzech metod otrzymywania KKP różniły się między sobą nie więcej niż o 1,65 punktu procentowego. Podobnie, zarówno mediany, jak i zakresy otrzymanych wyników serii były porównywalne. Wnioski. Powyższa analiza pozwala wnioskować, że na liczbę odzyskanych krwinek po ich rozmrożeniu nie miała wpływu metoda produkcji koncentratów krwinek płytkowych. Słowa kluczowe: koncentraty krwinek płytkowych, kriokonserwacja, rozmrażanie
Summary
Introduction. Platelet concentrates are essential for treating bleedings in patients with thrombocytopenia in order to prevent spontaneous hemorrhage. Platelets are also needed during invasive medical procedures. Storing their concentrates for further use can be prolonged by methods of cryopreservation. Aim. The aim of this study is to estimate whether platelet concentrates preparation methods affect the number of platelets recovered after cryopreservation. Material and methods. Analyses were carried out from June 2006 to January 2010. They included 160 platelet concentrates, received from aphaeresis and from pooled buffy coats. Platelets concentrates were frozen in five percent dimethyl sulfoxide (DMSO) and stored at -80°C. The number of platelets was counted before freezing and after thawing using the automated haematology analyser. Results. The average recovery of thawed platelets was 73.57% – varied from 72.77 up to 74.42% depending on the applied method. The analysis did not reveal statistically significant differences in recovery between platelets from particular groups. Mean values in particular groups varied by 1.65 per cent. Median values and scope of results for particular groups were similar. Conclusions. The above analysis proves that method of obtaining of platelet concentrate has no influence over the number of thawed platelets. Key words: platelet concentrates, cryopreservation, thawing
Introduction
Platelets are crucial for the process of blood clotting. Platelet count that is above and below normal may lead to various disturbances in homeostasis (1). After drawing and certain preparation, these cells are commonly used to treat platelet deficits. Transfusions are necessary in patients either with low platelet count or whose platelets show signs of functional disorders (2).
Main indications for platelet concentrate transfusions is treatment of bleedings occurring as a result of thrombocytopenia and preventing bleedings during invasive surgical procedures in patients with thrombocytopenia. Platelet concentrate is also administered prophylactically in hematologic diseases or before and after chemotherapy. Main indications for the transfusions are: hypoplastic anemia, acute leukemia, myelodysplastic syndromes, stem cell transplants. Platelet transfusions are performed in patients with functional disorders, such as: Glanzmann’s thrombasthenia, Bernard-Soulier syndrome and uremia. Other indications for platelet transfusions may be deficiencies connected to liver diseases or liver transplant (3-5).
Physiologically platelets’ lifespan is 8 to 12 days (6). Platelet concentrates are obtained by means of apheresis or from the stored blood (7). Storing platelets for transfusion purposes is difficult due to their sensitivity to changes in the environment. Storage at wrong temperature or pH changes cause change in the platelets’ discoid shape, as well as metabolic and functional disorders (6).
Changes in the cell’s cytoskeleton result in rearrangement in the structure of the cell membrane of both lipids and proteins. Mistakes in reception as well as transportation of the signals by the membrane receptors may induce unjustified activity and formation of microaggregates. On the other hand, changes in the cells’ metabolism prevent aggregation and adhesion. Normally, platelets’ shelf life is 5 to 7 days (after bacteriological tests).
Results of storing include drop in platelets count, “swirling”, changes in cells’ volume and shape, drop in pH and accumulation of lactate dehydrogenaze that constituses a cell lysis marker (8). Cryopreservation is an alternative storage method that significantly prolongs the platelets’ shelf life. Frozen platelet concentrate can constitute the reservoir in absence of fresh platelets (whose shelf life is 5 days).
The choice of freezing conditions should assure minimum water loss and prevent mechanical damage caused by formation of ice crystals inside the cells. Platelet concentrate must be frozen with the crioprotective to reduce the osmotic gradient between the intra- and intercellular space. To prevent recrystallization and deluting process, thawing should be relatively rapid (1).
Although there exist numerous methods of freezing and thawing of the platelets, loss in terms of quantity as well as function is more significant than in case of platelets stored in room temperature (6). Cryopreservation leads to drop in platelet count, lifespan in vivo as well as in vitro (8). Thawed platelets show inhibited aggregation in comparison with those stored in temperature of 22°C. However, their increased activity visible in changes in shape as well as expressed membrane receptors help in maintaining homeostasis (8-10).
Demand for platelet concentrates in the Regional Centre for Blood Donation and Haemotherapy in Katowice between 2005 and 2009 was from 7029 to 8856 therapeutic doses a year. Due to the constant demand for blood from all AB0 and Rh blood group systems, platelets were frozen and, if necessary, thawed and distributed to medical centres. Thawed platelet concentrates constituted 4.5-7.5% of all ordered platelet concentrate units (tab. 1, fig. 1).
Table 1. Platelets – mean recovery after thawing (% of the pre- freeze platelet content).
 Fig. 1. Platelets – recovery after thawing (%) – histogram (a – APHERESIS; b – OPTI; c – ORBISAC; d – cumulated results).
Aim
The purpose of this report is a quantitative analysis of platelets before and after thawing. The platelets were obtained by apheresis and buffy coat collection. The important question was whether the method of obtaining the platelet concentrate influences changes in the platelet count after the cryopreservation process.
Material and methods
The analysis was conducted from June 2006 to January 2010 and focused on 160 platelet concentrates stored in temperature of about -80°C for no longer than one year.
Platelets for production of pooled platelet concentrates were isolated from the buffy coats obtained from standard whole blood units. Four buffy coats were connected manually in sterile conditions, put in a container together with plasma and centrifuged.
Then platelet-rich plasma was separated with the press so that the number of platelets was above 2.4 x 1011 and the number of leukocytes was below 2 x 108. To obtain the pooled platelet concentrates automatically, OrbiSac system produced by Caridian BCT was used.
The number of platelets obtained exceeded 3 x 1011 and the number of leukocytes was below 1 x 106 due to the leukoreduction conducted during the production process. The third method of obtaining platelet concentrates was thrombopheresis performed with Haemonetics Cell Separator. The number of platelets in a sample from a single donor was above 3 x 1011.
Platelet concentrates were frozen with 100 ml of Dimethyl sulfoxide (DMSO) as a cryoprotective in final concentration of 5%. Containers with platelets were centrifuged for 9 minutes in 22°C at the speed of 5000 x g. DMSO was added to the part of plasma to obtain final concentration of 5%. After 20 minutes drops of the solution were added to the remaining plasma with platelets. Such concentrate was rapidly frozen by placing in the final temperature of -80°C.
The platelets were stored in a low temperature freezer at -80°C for a period of one month to one year.
Thawing was conducted in a dry thawing device under gentle stirring at of 37°C until the temperature of platelet concentrate rose to 22°C. To remove DMSO, drops of physiological salt solution enriched with vitamin C were added to the platelets. After centrifuging for 9 minutes at 5000 x g at a temperature of 22°C supernatant was removed. Plasma of the same type or from the same donor in case of platelets collected by apheresis was added to the concentrate.
Platelet count in the samples was measured with Sysmex K-4500 Automated Hematology Analyzer. The choice of tests conducted for the purposes of this report resulted from the standard quality control of the blood ingredient obtained, both before and after the freezing process.
160 therapeutic doses of platelet concentrate were tested before and after freezing. The tested material was divided into three basic groups according to the manner in which the platelets were isolated from the donor’s whole blood:
1. APHERESIS group – thrombopheresis, the method which uses Haemonetics Cell Separator (platelets drawn from one donor),
2. ORBISAC group – the automatic method of obtaining leucocyte-depleted platelet concentrates pooled from five buffy coats with OrbiSac System produced by Caridian,
3. OPTI group – manual method of obtaining platelet concentrates pooled from four buffy coats.
Tests results were presented as mean values ± SD (standard deviation). Results in all groups were compared by analysis of variance (ANOVA), test F with the use of Microsoft Excel software. The results were considered statistically significant at p ≤ 0.05.
Results
160 therapeutic units of platelet concentrate (42 obtained by APHERESIS method, 85 obtained by ORBISAC method and 33 by OPTI method) were tested before and after freezing. Platelet recovery after thawing for all groups was 72.42%. Standard deviation was at 0.74, which means the spread of the results around the mean value was significant.
The lowest recovery was at 46.0% and the highest at 96.34%, whereas the scope of results was within the norm i.e. > 40% of the initial value (tab. 2). After comparing the results of all groups, the highest mean recovery of 74.42% was recorded in platelet concentrate obtained by OPTI method.
Table 2. Platelets – Regional Center of Blood Donation and Treatment in Katowice production scale (2005-2008).
The results in this group varied from 59.4 to 90.3% with median value equal to the mean value. The percentage of platelets recovered after thawing by APHERESIS method was the lowest (72.38%). That group, however, had the greatest scope of results (from 61.4 to 88.6%) and the lowest standard deviation, which indicates greater uniformity of the material (tab. 2).
In the ORBISAC group, the platelet recovery after cryopreservation was at 73.53% and was placed between the two remaining results. After analyzing the scope and spread of the results in this group, the outliers were relatively distant from the mean value. The bar chart, however, presented the most regular results (fig. 2).
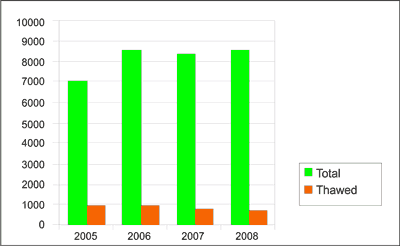 Fig. 2. Platelet demand (2005-2008).
The analysis did not reveal statistically significant differences in recovery between platelets from particular groups. Mean values in particular groups varied by 1.65 per cent. Median values and scope of results for particular groups were similar, as shown on a bar chart (fig. 2). The above analysis proves that method of obtaining of platelet concentrate has no influence over the number of thawed platelets.
Discussion
Freezing process, low temperature storing and reconstitution significantly influence the platelets count. After the analysis of 160 units collected before and after thawing, the mean recovery of platelets was at 72.4%. This result is similar to the results of other centres. Valeri applied the same method of cryopreservation and his mean platelet recovery after thawing was 75 ± 9%. The results were published in 2005 by Valeri and the co-authors (10). Later, the same author obtained mean platelet recovery of 74 ± 7% (11). Hornsey and others, obtained higher recovery values of 77 ± 9%. Results that are closest to those presented in this paper were obtained by Melaragno (75%) whose work was published by Hornsey (9).
The factor that influences platelet function to the greatest extent, is a method of preparation. Balint and others compared six methods of freezing taking into consideration the cell number, morphological changes and ability to aggregate. The authors applied the six-stage freezing model and rapid freezing with 6% DMSO, 10% DMSO and 5% DMSO with 6% HES (hydroxyethyl starch).
Methods which applied controlled freezing proved to be more effective in terms of all the measured parameters. The highest platelet recovery (90.96 ± 5%) was achieved in controlled freezing with 6% DMSO (1, 11). In December 2005 Valeri with co-authors published a work describing a new freezing method, which gave better results in terms of platelet count and function after thawing.
The difference of this method consisted in the fact, that the platelets suspended in plasma with 6% DMSO were centrifuged and the supernatant was aspirated. The cells suspended in a small amount of prepared solution were frozen (freezing temperature was controlled by the computer). Loss in platelet number was estimated at 23% (12). In the latest method of freezing the lyophilized platelets, which are cooled after thawing, the mean recovery was at 94 ± 2% (13).
An important aspect of the process is that after transfusion, the thawed platelets fulfill their function in case of bleedings, damage to the vessels or cell defects. The possible reaction to the hypotonic shock is increased activity that can be seen in the changes in shape, density of the cytoplasm, location of granules and organelles.
The platelets’ susceptibility to changes due to hypotonic shock depends on their age, shape and cell membrane integrity. It has been noticed that younger and larger cells are more resistant to changes caused by cryopreservation. The inactive platelets are discoid in shape. In activated platelets the arrangement of microtubules changes and the cells take spherical shape and form projections. Density of the cytoplasm decreases and granules migrate to the cell’s periphery (1).
Examining morphology of the thawed platelets, Balint noticed discoid shape in only half of the cells, whereas 15% had balloon-like appearance which proved irreversible damage to the cell membrane. Later the same author examined the interdependence between the thawed cells’ morphology and their lifespan. He concluded that the satisfactory in vivo lifespan can be expected if half of the transfused platelets preserve the discoid shape (11).
In their work Rothwell and others confirmed that thawed platelets are more susceptible to fragmentation and appearance of malformations. They also have tendency to adhesion and covering of the microscopic slide in vitro (8). The author determined that the biological half-life of the transfused fresh platelets was over 8 hours, whereas for the thawed ones it was only 2 hours (7).
In order to learn whether platelets stored in low temperature preserve the ability to aggregate, tests in other centres were conducted. They used aggregometer and the coagulation process was induced by ADP or thrombin. It was proved that after thawing the platelets’ ability to aggregate was decreased even several times (8, 10, 12). The drop in the pace of aggregation was also noticed. The reason is decrease in the cells’ ability for shape adjustment (8, 11).
Conclusions
Test results presented above indicate satisfactory recovery of the platelets after thawing and right choice of cryopreservation method. It may be confirmed by similar results provided by other authors. Method of platelet concentrates production proved to have no influence over the platelet recovery after cryopreservation. Piśmiennictwo
1. Balint B, Vucetic D, Trajkovic-Lakic Z et al.: Quantitative, functional, morphological and ultrastructural recovery of platelets as predictor for cryopreservation. Haematologia (Budap) 2002; 32(4): 363-375.
2. Herman JH: Przetaczanie koncentratów krwinek płytkowych. Leczenie krwią. Zasady postępowania klinicznego. Red Mintz PD Sekcja Transfuzjologiczna Pol Tow Hem i Transf, Warszawa 2001: 73-90.
3. Wandt H, Ehninger G, Gallmeier WM: New strategies for prophylactic platelet transfusion in patients with hematologic diseases. The Oncologist 2001; 6: 446-450.
4. Hayward C: Diagnostic approach to platelet function disorders. Transfus Apher Sci 2008; 38(1): 65-76.
5. Rebulla P: Revisitation of the clinical indications for the transfusion of platelet concentrates. Rev Clin Exp Hematol 2001; 5(3): 288-310.
6. Windyga J: Hemostaza fizjologiczna. [W:] Mariańska B, Fabiańska-Mitek J, Windyga J: Badania laboratoryjne w hematologii. PZWL, Warszawa 2006: 151-163.
7. Antoniewicz-Papis J, Dzieciątkowska A: Preparatyka krwi i jej składników. [W:] Łętowska M (red.): Medyczne zasady pobierania krwi, oddzielania jej składników i wydawania, obowiązujące w jednostkach organizacyjnych publicznej służby krwi. Instytut Hematologii i Transfuzjologii, Warszawa 2006: 1-101.
8. Rothwell SW, Maglasang P, Reid TJ et al.: Correlation of in vivo and in vitro functions of fresh and stored human platelets. Transfusion 2000; 40(8): 988-993.
9. Hornsey VS, McMillan L, Morrison A et al.: Freezing of buffy coat-derived, leukoreduced platelet concentrates in 6 percent dimethyl sulfoxide. Transfusion 2008; 48(12): 2508-2514.
10. Valeri CR, Macgregor H, Ragno G: Correlation between in vitro aggregation and thromboxane A2 production in fresh, liquid-preserved, and cryopreserved human platelets: effect of agonists, pH, and plasma and saline resuspension. Transfusion 2005; 45(4): 596-603.
11. Balint B, Paunovic D, Vucetic D et al.: Controlled rate versus uncontrolled rate freezing as predictors for platelet cryoprese rvation efficacy. Transfusion 2006; 46(2): 230-235.
12. Valeri CR, Ragno G, Khuri S: Freezing human platelets with 6 percent dimethyl sulfoxide with removal of the supernatant solution before freezing and storage at 80°C without postthaw processing. Transfusion 2005; 45(12): 1890-1898.
13. Zhou X, Zhu H, Zhang Sz et al.: Freeze-drying of human platelets: influence of saccharide, freezing rate and cell concentration. Cryo Letters 2007; 28(3): 187-196.
14. Lozano M: Consensus and controversies in platelet transfusion: trigger for indication, and platelet dose. Transfus Clin Biol 2007; 14(6): 504-508.
otrzymano/received: 2013-04-23 zaakceptowano/accepted: 2013-06-07 Adres/address: *Joanna Bienias Regional Center of Blood Donation and Treatment ul. Raciborska 15, 40-076 Katowice tel.: +48 663-101-236 e-mail: abienias3@op.pl Artykuł Ocena krwinek płytkowych po procesie kriokonserwacji w Czytelni Medycznej Borgis. |
|||||||||||||||||||||||||||||||||||||||||||||||
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








