|
© Borgis - Postępy Nauk Medycznych 10, s. 771-776
Małgorzata Drobnicka-Stępień1, Joanna Narbutt2, Irmina Olejniczak2, Anna Sysa-Jędrzejowska2, *Aleksandra Lesiak2
Niskie stężenie kwasu foliowego jako jeden z czynników predysponujących do rozwoju raków podstawnokomórkowych skóry
Low folic acid serum concentration as one of the factors leading to basal cell carcinoma development**
1Infectious and liver diseases ward, Bieganski Hospital, Łódź
Head of Department: prof. Zbigniew Deroń, MD, PhD 2Department of Dermatology, Medical University of Łódź Head of Department: prof. Anna Sysa-Jędrzejowska, MD, PhD Streszczenie
Rak podstawnokomórkowy skóry jest najczęściej występującym nowotworem wśród ludzi rasy białej, stanowi 80% nowo zdiagnozowanych guzów. Niedobór kwasu foliowego został ostatnio uznany za czynnik ryzyka w przypadku kilku nowotworów złośliwych. Cel. Ocena roli metabolizmu kwasu foliowego w procesie karcynogenezy u pacjentów z rakiem podstawnokomórkowym skóry poprzez oznaczenie stężenia kwasu foliowego w surowicy pacjentów chorych na raka podstawnokomórkowego i w grupie kontrolnej. Materiały i metody. Grupa doświadczalna liczyła 125 pacjentów rasy białej, 79 osób (41 kobiet i 38 mężczyzn, średnia wieku – 60,2 lata, fototyp I/II – 20, III – 52, IV – 7) ze zdiagnozowanym na podstawie badania histopatologicznego rakiem podstawnokomórkowym skóry oraz 46 zdrowych ochotników (21 kobiet, 25 mężczyzn, średnia wieku – 58,4 lata. fototyp I/II – 10, III – 28, IV – 8). U wszystkich pacjentów pomiar stężenia kwasu foliowego w surowicy został przeprowadzony za pomocą Vitamin Folic Acid Test (DRG Vitamin Folic Acid, Mountainside, USA). Wyniki. Stężenie kwasu foliowego było znacząco wyższe w grupie kontrolnej niż w grupie pacjentów z rakiem podstawnokomórkowym skóry (odpowiednio: mediana 16,5 μg/l vs. mediana 9,6 μg/l; p < 0,001). U większości badanych zarówno z grupy kontrolnej, jak i z grupy chorych na raka podstawnokomórkowego skóry stężenie kwasu foliowego w surowicy mieściło się w normalnych granicach. Wnioski. Na podstawie otrzymanych wyników i danych zawartych w literaturze możemy stwierdzić, że kwas foliowy bierze udział w rozwoju raka podstawnokomórkowego skóry i jego niedobór może być uznany za jeden z czynników zwiększających ryzyko wystąpienia karcynogenezy skóry. Słowa kluczowe: kwas foliowy, rak podstawnokomórkowy skóry, karcynogeneza
Summary
Basal cell carcinoma (BCC) is the most common neoplasm in Caucasian population, it represents over 80% of newly diagnosed tumors. Folic acid insufficiency has been recently considered as a risk factor for several cancers. Aim. To assess the contribution of folic acid metabolism in the process of carcinogenesis in patients with BCC by determining the concentration of folic acid in the serum of patients with BCC and in control group. Material and methods. Study group included 125 Caucasian subjects, 79 persons (41 women, 38 men, median age – 60.2 years, phototype: I/II – 20, III – 52, IV – 7) with BCC diagnosed on the basis of histopathological examination and 46 healthy volunteers (21 women, 25 men, median age – 58.4 years, phototype: I/II – 10, III – 28, IV – 8). In all patients serum folic acid concentration was measured with the use of Vitamin Folic Acid Test (DRG Vitamin Folic Acid, Mountainside, USA). Results. Folic acid concentration was significantly higher in a control group than in BCC patients (median 16.5 μg/l vs. median 9.6 μg/l; respectively; p < 0.001). In most of the subjects both from control group and with BCC folic acid serum concentration was within normal limit. Conclusions. Based on the obtained results and literature data we may conclude that the folic acid is involved in BCC development and its insufficiency may be concerned as one of the risk factors leading to skin cancerogenesis. Key words: folic acid, basal cell carcinoma, cancerogenesis
Recently increase in frequency of non-melanoma skin cancers (NMSC) which include basal cell carcinomas (BCC) and squamous cell carcinomas (SCC) has been observed (1-3). Basal cell carcinoma is the most common neoplasm in Caucasians and in Australian population and it represents over 80% of newly diagnosed cancers (4). In white race its frequency estimates between 18 and 40% (5, 6).
Despite of low mortality, NMSC, as the most common tumors in USA, Europe and Australia, are the major medical, social and economic problem (5, 7, 8).
Folic acid is a complex of folates, among which pteroil-1glutamic acid is the most stable form, therefore it is used in diet supplements, while it is rarely found in nature. Folates are sensible to high temperature, sun radiation and low pH. The active folates in the organism that act as coenzymes in many metabolic reactions are 5-tetrahydrofolate derivatives. They transfer one-carbon units in synthesis of purine and pyrimidine nucleotides, are involved in the synthesis of deoxyribonucleic acid (DNA) and therefore are essential for the correct cell division. They also play an important role in the metabolism of amino acids. One of the major reaction is the methylation of homocysteine to methionine – an amino acid which is an important substrate for the methylation reactions (9). Methionine derived from food undergoes remethylation into homocysteine. Disconnected methyl group is used for methylation of various compounds such as phospholipids, proteins, DNA and RNA. Approximately 50% of homocysteine is converted with the participation of vitamin B6, to cysteine. The remaining 50% is remethylated to methionine. 5-methylenetetrahydrofolate and vitamin B12 are necessary for this reaction (10) (fig. 1).
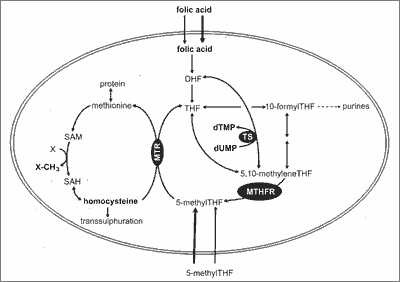 Fig. 1. Folate metabolic pathway (9).
The active form of folic acid (5-methylenetetrahydrofolate) is involved in the synthesis of purines, pyrimidines and DNA synthesis, amino acid metabolism and in the synthesis and transformation of formates. It also plays an important role in tissues with high rates of cell division, especially in the hematopoietic system, gastrointestinal tract epithelia and fetal tissues. In addition, it is important in the process of myelination of nerve fibers. During pregnancy it prevents neural tube birth defects in the fetus (11, 12).
The role of folate in preventing the development of cancer is not fully elucidated. Epidemiological studies suggest an inverse relationship between folate intake and the occurrence of cancer of the colon, lung, pancreas, esophagus, stomach, cervix, prostate, ovarian, breast cancer and leukemia (13, 16).
Most of data concerns the role of folic acid in preventing colon cancer. Recent studies showed an inverse relationship between folate intake or blood folate levels and risk of colorectal cancer. The postulated link between folate deficiency and carcinogenesis is likely due to the participation of this vitamin in the synthesis of DNA. Folate deficiency is responsible for impairment of DNA methylation, increased chromosome fragility and decreased ability to repair damaged DNA fragments, which contributes to mutagenesis (15, 17-19).
There are only scarce data on the role of folate insufficiency in BCC development. Thus, the aim of the study was to assess the contribution of folic acid metabolism in the process of carcinogenesis in patients with BCC by determining the concentration of folic acid in the serum of patients with BCC and in control group.
Material and methods
Study group consisted of 125 Caucasian subjects, including 79 (41 women, 38 men, median age – 60.2 years, phototype: I/II – 20, III – 52, IV – 7) persons with BCC diagnosed on the basis of histopathological examination and 46 healthy volunteers (21 women, 25 men, median age – 58.4 years, phototype: I/II – 10, III – 28, IV – 8). The control group was randomly selected (tab. 1). The inclusion criterion was negative history for any neoplasms. Exclusion criteria were the use of tanning bath or increased exposure to sunlight within two months prior the study. Patients with BCC were treated in Outpatient Clinic of Dermatology and Venereology Medical University of Łódź between 2005 and 2008. None of the them was transplant recipient, was treated with immunosupresants, nor suffered from internal organs neoplasm. In all the subjects risk factors for BCC development were evaluated. They included lesion localization, chronic sunlight exposure, using of sunbeds, past history of sunburn, smoking, alcohol abuse. The patients’ skin types were defined according to the Fitzpatrick classification (1988). From all patients blood samples in order to determine serum folic acid concentration, were taken. Sampled sera were stored at -25°C until the measurement. Measurement of the total folic acid serum level was performed with the use of Vitamin Folic Acid Test (DRG Vitamin Folic Acid, Mountainside, USA). This assay is a microtiter plate test kit based on a microbiological assay. Serum samples were diluted with a buffer solution. The diluted samples were added into the microtiter plate wells [coated with Lactobacillus rhamnosus which metabolizes folic acid]. The addition of folic acid in either standards or samples gave a folic acid-dependent growth response until it was consumed. After incubation at 37°C for 48 h, the growth of Lactobacillus rhamnosus was measured turbidimetrically at 610-630 nm (alternative at 540-550 nm) in an ELISA-reader and a standard curve was generated from the dilution series. The amount of folic acid was directly proportional to the turbidity.The reference values of folate concentrations in this method were 3.8-23.2 ug/l. The obtained results were statistically evaluated with the use of STATISTICA 6.0 Software (Statsoft, Tulusa, USA).
Table 1. Clinical characteristic of BCC patients and control group.
Results
In most cases (n = 67, 84.8%) basal cell carcinomas were located on the body areas exposed to sunlight (face, neck, dorsal side of hands), only in 12 (15.2%) cases tumor was located on unexposed surfaces (back, lower limbs). In 47 patients (59.5%) there was significant medical history concerning sunburn, in 68 subjects (86.1%) – erythema after sun exposure. In analyzed BCC-group 49 patients (62.0%) suffered had at least 1 incidence of sunburn, while 30 (38.0%) did not notice this side effect after sunlight exposure. 53.2% of patients (n = 42) were smoking.
Family history of cancers was positive in 40 patients (50.6%). Family incidence of cancer has been confirmed by 41 patients (51.9%), while 38 (48.1%) had a negative family history in terms of skin cancer. 42 respondents (53.1%) work in the open-air, while 37 patients (46.8%) practiced indoor.
Folic acid serum concentration was significantly higher in a control group than in BCC patients (median 16.5 μg/l (min 4.6 μg/l – max 70.6 μg/l) vs median 9.6 μg/l (min 3.4 μg/ml – max 30.6 μg/l; p < 0.001) (fig. 2). In most of the subjects both from control group and with BCC folic acid serum concentration was within normal limits. We found no correlation between folic acid serum level and phenotypic features such as sex, age, skin phototype, hair and eyes colour (p > 0.05 for all comparison).
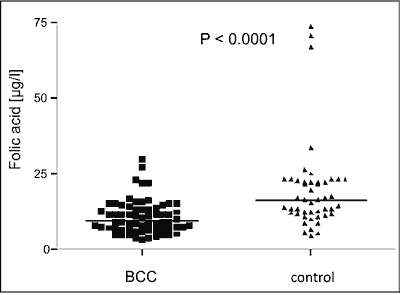 Fig. 2. Folic acid serum concentration in BCC patients and in healthy control.
Discussion
Recently dynamic increase in skin cancers occurrence has been observed. They account for about 10% of all cancers in men, 90% of them are NMSC skin cancers, most of which are basal cell carcinomas (approx. 80%) (20).
Based on studies conducted between 1973-2000 in several countries an increase in BCC incidence in both men and women was noted (from 40 to 92 cases of BCC per 100 000 men and from 34 to 79 cases per 100 000 women) (21).
The fact of dynamic increase in BCC incidence in recent decades has contributed to conducing of numerous studies aimed at understanding the phenomena underlying the pathogenesis of cutaneous photocancerogenesis, as well as interactions between environmental factors and molecular genetics.
Clinical observations indicate that some factors may predispose to the development of BCC. These include, among others, age, gender, occupation and environmental factors. It seems that their individual participation may vary by region of the world and as it may change over the years.
In the group of patients there was no difference between the incidence of BCC and gender, which is in agreement with literature data, indicating that the difference between the occurrence of cancer in each sex is no longer significant. The confirmation of these findings are the results of studies conducted in European, Australian, and Asian populations (22-24). Previous studies have indicated a higher incidence of BCC in men. Nowadays, an increasing number of new cases in the female has been observed (25-28). This may be due to more frequent use of sunbeds by women and even, in some cases, addiction to artificial light sources (21, 29). It is widely recognized that there is a higher prevalence of BCC in people between seven and eight decade of life (30). However, recently decrease in average age of onset of BCC, from 56 to 30-39 years, has been noted (31). It is probably caused by observed also in our country, change of lifestyle, more frequent sun exposure and the usage of artificial radiation sources (31, 32). One of the proofs for the participation of tanning in sunbeds is in BCC development is its increase of the localization in the body area normally not exposed to sunlight, for example on the trunk (21, 29). However in our own study, in most cases (n = 67, 84.8%) BCC were located on the skin exposed to the sun (face, neck, dorsal side of hands) and in only 12 (15.2%) of patients had tumor in not exposed areas (trunk, lower limbs).
Literature data also show that the majority of cases of BCC localize on the scalp and neck (80%), and only 15% of them on the body (33). Scrivener al. (2002) anlysed 13 457 cases of BCC, most of which occurred on the skin of the head (89.6%), while significantly fewer cases were found on the rest of the body (34).
Analyzing the dependence of the incidence of BCC on the race it has been shown that the Caucasian race is particularly predisposed to skin cancerogenesis because of fair skin and low skin phototype. These are the features that promote development of cutaneous photocancerogenesis and explain an increased risk of skin cancers among Caucasians (35-37).
In most BCC patients (80%) low skin phototype (light hair and eye color, tendency to sunburn) was noted. Most of the subjects, 49 (62.0%) reported the occurrence of sunburn in the past, while 30 (38.0%) persons did not notice this side effect after exposure to solar radiation.
Over half of the patients gave the history of frequent sunbath, and almost 90% of them confirmed the presence of erythema after exposure to UVR. These data confirm that ultraviolet radiation is one of the most important environmental factors involved in skin carcinogenesis (38, 39).
Normal serum folate concentration is essential in physiological conditions to repair DNA damage caused by ultraviolet radiation. Thus, folates and their metabolites are vital for normal cell proliferation and DNA repair in rapidly dividing cells which include keratinocytes. Folates are precursors of S-adenozynomethionine, which is essential in de novo synthesis of substrates involved in the processes of DNA replication and repair. The most common epigenetic phenomenon in the process of carcinogenesis is incorrect DNA methylation (40, 41). DNA methylation is an important process for normal cell division and development. The impairment of the process occurs with aging and in the course of cancerogenesis.
The process of methylation plays also a key role in regulating gene expression and maintaning genome integrity. Decreased concentrations of folic acid cause disruption in the availability of nucleotides, incorrect incorporation of uracil in the DNA what in a consequence leads to impaired replication and DNA strand break (42, 47).
Thus, folate insufficiency is associated with the development of some cancers, which contributed to the hypothesis that folic acid supplementation may be a preventive approach to their development (48). Confirmation of this hypothesis are the study results obtained by Zhang (49) and Hussien et al. (50) who showed that folic acid supplementation significantly reduces the risk of developing breast cancer, especially in alcohol abuse women.
In a recently published study, Liang et al. (51) showed the presence of DNA hypomethylation in skin squamous cell carcinomas, what indirectly justifies the prophylactic use of folate supplementation.
Our results and literature data confirm the participation of folate metabolism in the pathogenesis of cutaneous cancerogenesis.
To our best knowledge there is no other data on folic acid serum level in BCC patients. Our study showed significantly lower folic acid concentrations in patients with BCC (9.6 μg/l) compared to the control group (16.5 μg/l), although in all cases they were within normal limits.
Based on the obtained results and literature data we can not clearly define the role of folic acid in the development of basal cell carcinoma, although there are some strong proofs for its significant participation in the process of skin cancerogenesis.
**The project was funded by the Medical University of Łódź Research Grant number 503-1152-1 and the Polish Scientifique Committee Grant number NN402474731. Piśmiennictwo
1. Lear JT, Smith AG: Basal cell carcinoma. Postgrad Med J 1997; 73: 538-542.
2. Lear JT, Tan BB, Smith AG et al.: Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med 1997; 90: 371-374.
3. Trakatelli M, Ulrich C, del Marmol V et al.: Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol 2007; 156: 1-7.
4. Katalinic A, Kunze U, Schäfer T: Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer). Br J Dermatol 2003; 149: 1200-1206.
5. English DR, Kricker A, Heenan PJ et al.: Incidence of non-melanocytic skin cancer in Geraldton, Western Australia. Int J Cancer 1997; 73: 629-633.
6. Wong CS, Strange RC, Lear JT: Basal cell carcinoma. BMJ 2003; 327:794-798.
7. Miller DL, Weinstock MA: Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol 1994; 30: 774-778.
8. Richmond-Sinclair NM, Pandeya N, Ware RS et al.: Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol 2009; 129: 323-328.
9. Wagner C: Symposium on the subcellular compartmentation of folate metabolism. J Nutr 1996; 126 (4 Suppl.): 1228S-1234S.
10. Tam TT, Juzeniene A, Steindal AH et al.: Photodegradation of 5-methyltetrahydrofolate in the presence of uroporphyrin. J Photochem Photobiol B 2009; 94: 201-204.
11. Wolff T, Witkop CT, Miller T, Syed SB: Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Update of the Evidence for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2009 May.
12. Bodnar LM, Himes KP, Venkataramanan R et al.: Maternal serum folate species in early pregnancy and risk of preterm birth. Am J Clin Nutr 2010; 92: 864-871.
13. Charles D, Ness AR, Campbell D et al.: Taking folate in pregnancy and risk of maternal breast cancer. BMJ 2004; 329: 1375-1376.
14. Hultdin J, Van Guelpen B, Bergh A et al.: Plasma folate, vitamin B12, and homocysteine and prostate cancer risk: a prospective study. Int J Cancer 2005; 113: 819-824.
15. Sanjoaquin MA, Allen N, Couto E et al.: Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer 2005; 113: 825-828.
16. Liu JJ, Ward RL: Folate and one-carbon metabolism and its impact on aberrant DNA methylation in cancer. Adv Genet 2010; 71: 79-121.
17. Giovannucci E, Chan AT: Role of vitamin and mineral supplementation and aspirin use in cancer survivors. J Clin Oncol 2010; 28: 4081-4085.
18. Giovannucci E, Rimm EB, Ascherio A et al.: Alcohol, low-methionine-low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst 1995; 87: 265-273.
19. Giovannucci E, Stampfer MJ, Colditz GA et al.: Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst 1993; 85: 875-884.
20. Nasser N: Epidemiology of basal cell carcinomas in Blumenau, SC, Brazil, from 1980 to 1999. An Bras Dermatol 2005; 80: 363-368.
21. de Vries E, Louwman M, Bastiaens M et al.: Rapid and continuous increases in incidence rates of basal cell carcinoma in the southeast Netherlands since 1973. J Invest Dermatol 2004; 123: 634-638.
22. Czarnecki D, Zalcberg J, Meehan C et al.: Familial occurrence of multiple nonmelanoma skin cancer. Cancer Genet Cytogenet 1992; 61: 1-5.
23. Cho S, Kim MH, Whang KK, Hahm JH: Clinical and histopathological characteristics of basal cell carcinoma in Korean patients. J Dermatol 1999; 26: 494-501.
24. Beattie PE, Finlan LE, Kernohan NM et al.: The effect of ultraviolet (UV) A1, UVB and solar-simulated radiation on p53 activation and p21. Br J Dermatol 2005; 152: 1001-1008.
25. Ceylan C, Oztürk G, Alper S: Non-melanoma skin cancers between the years of 1990 and 1999 in Izmir, Turkey: demographic and clinicopathological characteristics. J Dermatol 2003; 30: 123-131.
26. Raasch BA, Buettner PG, Garbe C: Basal cell carcinoma: histological classification and body-site distribution. Br J Dermatol 2006; 155: 401-407.
27. Neale RE, Davis M, Pandeya N et al.: Basal cell carcinoma on the trunk is associated with excessive sun exposure. J Am Acad Dermatol 2007; 56: 380-386.
28. Coups EJ, Manne SL, Heckman CJ: Multiple skin cancer risk behaviors in the U.S. population. Am J Prev Med 2008; 34: 87-93.
29. Marehbian J, Colt JS, Baris D et al.: Occupation and keratinocyte cancer risk: a population-based case-control study. Cancer Causes Control 2007; 18: 895-908.
30. Egeler RM, Favara BE, van Meurs M et al.: Differential In situ cytokine profiles of Langerhans-like cells and T cells in Langerhans cell histiocytosis: abundant expression of cytokines relevant to disease and treatment. Blood 1999; 94: 4195-4201.
31. Bath-Hextall F, Leonardi-Bee J, Smith C et al.: Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. Int J Cancer 2007; 121: 2105-2108.
32. Faurschou A, Wulf HC: Ecological analysis of realation between sunbeds and skin cancer. Photodermatol Photoimmunol Photomed 2007; 4: 120-125.
33. Lovatt TJ, Lear JT, Bastrilles J et al.: Associations between ultraviolet radiation, basal cell carcinoma site and histology, host characteristics, and rate of development of further tumors. J Am Acad Dermatol 2005; 52: 468-473.
34. Scrivener Y, Grosshans E, Cribier B: Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol 2002; 147: 41-47.
35. Daya-Grosjean L, Dumaz N, Sarasin A: The specificity of p53 mutation spectra in sunlight induced human cancers. J Photochem Photobiol B 1995; 28: 115-124.
36. Garssen J, van Loveren H: Effects of ultraviolet exposure on the immune system. Crit Rev Immunol 2001; 21: 359-397.
37. Diffey BL: Sources and measurement of ultraviolet radiation. Methods 2002; 28: 4-13.
38. Żak-Prelich M, Sysa-Jędrzejowska A, Narbutt J: Environmental risk factors predisposing to the development of basal cell carcinoma. Dermatologic Surgery 2004; 30: 248-252.
39. Narbutt J, Lesiak A, Ekiert A, Sysa-Jędrzejowska A: Environmental factors in nonmelanoma skin cancer development. Polish J Environ Studies 2005; 14: 545-550.
40. Duthie SJ: Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull 1999; 55: 578-592.
41. Das PM, Singal R: DNA methylation and cancer. J Clin Oncol 2004; 22: 4632-4642.
42. Cheah MS, Wallace CD, Hoffman RM: Hypomethylation of DNA in human cancer cells: a site-specific change in the c-myc oncogene. J Natl Cancer Inst 1984; 73: 1057-1065.
43. Wainfan E, Dizik M, Stender M, Christman JK: Rapid appearance of hypomethylated DNA in livers of rats fed cancer promoting, methyl-deficient diets. Cancer Res 1989; 49: 4094-4097.
44. Branda RF, Blickensderfer DB: Folate deficiency increases genetic damage caused by alkylating agents and gamma-irradiation in Chinese hamster ovary cells. Cancer Res 1993; 53: 5401-5408.
45. Sancar A: Mechanism of DNA excision repair. Science 1994; 266: 1954-1956.
46. Jacob RA, Gretz DM, Taylor PC et al.: Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. J Nutr 1998; 128: 1204-1212.
47. Lajous M, Lazcano-Ponce E, Hernandez-Avila M et al.: Folate, vitamin B(6), and vitamin B(12) intake and the risk of breast cancer among Mexican women Cancer Epidemiol Biomarkers Prev 2006; 15: 443-448.
48. Kim YI: Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer. Cancer Epidemiol Biomarkers Prev 2004; 13: 511-519.
49. Zhang SM: Role of vitamins in the risk, prevention and treatment of breast cancer. Curr Opin Obstet Gynecol 2004; 16: 19-25.
50. Hussien MM, McNulty H, Armstrong N et al.: Investigation of systemic folate status, impact of alcohol intake and levels of DNA damage in mononuclear cells of breast cancer patients.Br J Cancer 2005; 92: 1524-1530.
51. Laing ME, Cummins R, O’Grady A et al.: Aberrant DNA methylation associated with MTHFR C677Tgenetic polymorphism in cutaneous squamous cell carcinoma in renal transplant patients. Br J Drmatol 2010; 163: 345-352.
otrzymano/received: 2012-08-22 zaakceptowano/accepted: 2012-09-28 Adres/address: *Aleksandra Lesiak Department of Dermatology Medical University of Łódź ul. Krzemieniecka 5, 94-014 Łódź tel.: +48 (42) 686-79-81 e-mail: aleksandra.lesiak@umed.lodz.pl Artykuł Niskie stężenie kwasu foliowego jako jeden z czynników predysponujących do rozwoju raków podstawnokomórkowych skóry w Czytelni Medycznej Borgis. |
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








