|
© Borgis - Postępy Nauk Medycznych 8, s. 668-676
*Włodzimierz Hendiger, Zbigniew Kwietniak, Adam Lewszuk, Walerian Staszkiewicz
Możliwości i ograniczenia w endowaskularnym zaopatrzeniu tętniaka aorty brzusznej
Possibilities and limitations in the endovascular repair of abdominal aortic aneurysm
Department of Vascular Surgery and Angiology of the Medical Centre for Postgraduate Education, The Jerzy Popiełuszko Memorial Bielański Hospital
Head of Department: prof. Walerian Staszkiewicz, MD, PhD Streszczenie
Tętniak aorty brzusznej (TAB) jest przyczyną od 1 do 3% zgonów wśród mężczyzn pomiędzy 65 i 85 rokiem życia. Rejestruje się w ciągu ostatnich dwudziestu lat wzrost zachorowań na tętniak co może być związane ze starzeniem się społeczeństwa, wzrostem liczby palaczy ale także z wprowadzeniem programów przesiewowych i stałym unowocześnieniem metod diagnostycznych. Pacjenci z bezobjawowymi tętniakami o średnicy od 55 mm kwalifikowani są do elektywnego zabiegu wyłączenia tętniaka celem zmniejszenia ryzyka pęknięcia. Dostępne są dwie metody zabiegowe: metodą otwartej laparatomii oraz metodą wszczepienia wewnętrznej protezy (tak zwanego stentgraftu) od strony tętnic udowych bez otwierania jamy brzusznej. Wszczepienie stentgraftu przeprowadzane jest wewnątrznaczyniowo (EVAR). Celem pracy jest wybór metody postępowania, kwalifikacja do metody otwartej lub wewnątrznaczyniowej leczenia tętniaków aorty brzusznej. Planowy zabieg wewnątrznaczyniowy 2-3 krotnie zmniejsza 30 dniową śmiertelność pooperacyjną w stosunku do operacji klasycznej. EVAR w stosunku do klasycznej operacji jest procedurą małoinwazyjną polegającą na wewnątrznaczyniowy wprowadzeniu specjalnej protezy (stentgraftu) do jamy tętniaka z dostępu przez tętnice udowe. Nawet jeżeli EVAR łączy się z większym odsetkiem reinterwencji późnych to jest leczeniem chętniej akceptowanym przez pacjentów. Wewnątrznaczyniowa metoda zaopatrzenia tętniaka staje się metodą z wyboru. Słowa kluczowe: tętniak aorty brzusznej, operacja, wewnątrznaczyniowe zaopatrzenie tętniaka, stentgraft
Summary
The abdominal aortic aneurysm (AAA) is the cause of 1 to 3% of deaths among males in the age between 65 and 85 years. Within the last twenty years, increased incidence of aneurysms was recorded, which may be associated with population aging, and increasing number of smokers, but also with introduction of screening programs and constant modernization of diagnostic methods. Patients with asymptomatic AAA measuring at least 55 mm in diameter are classified to elective surgery in order to reduce the risk of aneurysm rupture. Two treatment methods are available: an open laparotomy and implantation of an internal prosthesis (so-called stent graft) through the femoral artery approach without opening the abdominal cavity. Stent graft is implanted with the endovascular method (EVAR). Purpose of this study is to select treatment methods, and qualification for the open laparotomy or endovascular treatment of the abdominal aortic aneurysms. Comparing to standard surgery, there is two- to three-fold reduction of 30-day postoperative mortality in the elective endovascular treatment. EVAR, comparing to the classic surgery, is a minimally invasive procedure involving the introduction of a special endovascular prosthesis (stent graft) into the aneurysm cavity through the femoral artery approach. Even if EVAR is associated with higher rates of late repeated interventions, it is more willingly accepted by the patients. Endovascular aortic repair becomes the method of choice. Key words: aortic aneurysm abdominal, surgery, EVAR, stent graft
Introduction
The abdominal aortic aneurysm (AAA) is the cause of 1 to 3% of deaths among males in the age between 65 and 85 years. Usually, the aneurysm is asymptomatic, unless its rupture occurs, which in 65-80% of case results in death (1). The abdominal aortic aneurysms are usually detected by accident during ultrasound evaluation of the abdominal cavity due to general surgical diseases or urological diseases. Within the last twenty years, increased incidence of aneurysms was recorded, which may be associated with population aging, and increasing number of smokers, but also with introduction of screening programs and constant modernization of diagnostic methods. Significance of the ultrasound screening methods in order to diagnose AAA is emphasized (2). This evaluation is performed by trained personnel (technicians, nurses) using ultrasound machine. The study focuses on the abdominal segment of the aorta. Longitudinal and transverse imaging of the aorta should be performed (3). The aneurysm is diagnosed if the diameter of the abdominal aorta exceeds 30 mm. Measurement is performed outside of the aorta. If the measurement is performed within the lumen of the aorta, then actual size is larger by thickness of the wall, i.e. by 2-5 mm, provided that there is no clot in the lumen. Studies demonstrate that the most beneficial screening in form of decreased percentage of recorded ruptured aneurysms is achieved if the screening is conducted in male patients in the age between 65 and 85 years, especially in smokers as well as persons with congenital burden (4). In case of stable and asymptomatic aneurysms, their treatment method mainly depends on their size. Aneurysms measuring over 55 mm are qualified for the elective surgery. Studies on small aneurysms revealed no benefits from conducted surgical treatment in case of aneurysms measuring less than 55 mm in diameter (5). In case of aneurysms below this size, the risk of surgery exceeds the risk of the aneurysm rupture. The patients with asymptomatic aneurysms measuring at least 55 mm in diameter are qualified for the elective surgery of the aneurysm elimination in order to reduce the risk of rupture. Currently, two surgical methods are available: an open laparotomy and implantation of an internal prosthesis (so-called stent graft) from the femoral artery approach without opening the abdominal cavity. Stent graft is implanted with the endovascular method (EVAR). This method is used if performing classic laparotomy would overburden the patient. Performing classic laparotomy may not be possible due to difficult anatomy of the aneurysm, or it may be associated with the risk of complications such as leakages, thrombosis in the prosthesis, movement of the prosthesis or its damaging.
Purpose of this study is selection of treatment methods, and qualification for the open laparotomy or endovascular treatment of the abdominal aortic aneurysms.
Definition
The expression “aneurysm” (aneurysma) comes from Greek word ανευρυσμα.
It means dilation of the artery lumen. The first description of the aneurysm comes from the 2nd century B.C. from Roman physicians, Antyllos and Galen. They described pulsating tumors occurring after the vessel injury.
The abdominal aortic aneurysm is formed by saccular or fusiform dilation of the aorta lumen by 50% in its abdominal segment comparing to diameter of normal vessel located above (6, 7). Currently, after analysis of data provided by CT-scan (fig. 1), it is assumed that diameter of normal aorta in the abdominal segment, below the renal artery, in adult male is 19.9 mm ± 2.2 mm (ranging from 15 to 24 mm) (8), and in females it is smaller by 2-3 mm. The diameter depends on age, gender and body weight (9). Therefore, the abdominal aortic aneurysm means dilation of the aorta, which is exceeding 30mm in diameter (10-12).
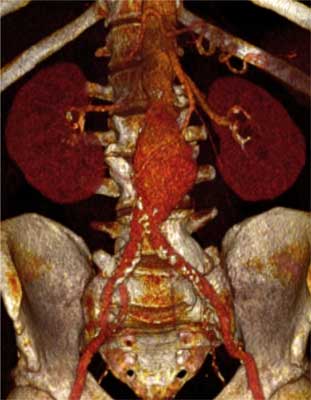 Fig. 1. Image of 3D reconstruction of the abdominal aortic aneurysm in the computerized tomography angiogram.
95% of aneurysms of the abdominal segment of the aorta is located below the renal arteries. It should be remembered that in 12% of the patients, the abdominal aortic aneurysm is also accompanied by aneurysms in other locations (13): e.g. iliac, popliteal. For this reason, it is important during diagnostics to pay attention at other segments of the arteries, not only at the abdominal segment.
Image of the abdominal aortic aneurysm in CT-scan is presented in figure 1.
Epidemiology
Extended life expectancy in population, and first of all, dynamic technological progress and availability of the minimally invasive ultrasound diagnostics, computerized tomography and magnetic resonance imaging, resulted in increase in detection of the abdominal aortic aneurysms (14-17). It is believed that sensitivity of the magnetic resonance imaging in detection of the abdominal aortic aneurysms is 100% (18). Within the period from 1951 to 1980, there was nearly seven-fold increase in number of the patients treated due to the aneurysm in Mayo Clinic, which clearly indicates significance of this fact (19).
Multicenter studies conducted on large groups of patients allow estimating incidence of the aneurysms in populations and risk factors favorable for their development. The prospective study Honolulu Hart, which was the program lasting over 20 years, and conducted on the group of 7682 males showed the following: the aortic aneurysm was diagnosed in 151 persons, and in 138 cases it was located in the abdominal segment. Risk factors in this group of the patients included increased blood pressure, increased cholesterol level in serum and smoking (20). The next program conducted in 1992-1995, Aneurysm Detection and Management (ADAM), included 15 centers (Veterans Affairs Medical Administration). In total, 73 451 subjects in the age of 50-79 years were studied. The aortic aneurysm was diagnosed in 1.4% of cases. Risk factors included hypercholesterolemia, hypertension, smoking to a large extent, and age as well as occurrence of this disease in a family history (21). The screening was also conducted in elderly male patients in multicenter study MASS (The Multicenter Aneurysm Screening Study). The group included 67 800 males in the age between 65 and 74 years. After randomization, ultrasound evaluation was performed in 33 839 subjects. Among persons, who underwent ultrasound evaluation, the abdominal aortic aneurysm was diagnosed in 1333 patients, which is 3.9% of subjects qualified for ultrasound evaluation and 1.9% of all males enrolled into the study. This study also demonstrated that 65 patients died due to rupture of the aneurysm (22). The Cardiovascular Heath Study including 4 populations living in the United States, studied 4734 males and females in the age of 65 years, and the abdominal aortic aneurysm was diagnosed in 8.8% of subjects, and it was more common in elderly males burdened with smoking, heart diseases, and hyperlipidemia. This study also revealed interrelation between occurrence of the abdominal aortic aneurysm and increased thickness of the wall of the carotid artery.
Aforementioned data shows how important is diagnostics and search for early factors of development of the aortic diseases in order to start treatment as early as possible.
Pathophysiology of the aneurysm development
The main cause for the abdominal aortic aneurysm development is functional and structural loss of the elastin in the aortic wall. This process has not been fully explained. Below, some processes will be discussed, which may lead to the aneurysm development.
Influence of the paramural clot
The paramural clot develops within the lumen of the aneurysm. It is associated with disturbed, irregular, turbulent flow in the dilated vessel. Usually, the clot has layered structure as a result of its gradual accumulation. The clot itself does not decrease pressure acting on the aortic wall, but only creates more even distribution of forces acting in the aneurysm. Computer analysis reveals that in case of presence of the clot, the highest tension occurs at its edges (23). It does not reduce the risk of the aneurysm rupture. The clot leads to ischemia of the aortic wall by impaired diffusion of the nutritional facts and to accumulation of the inflammatory cells within the space near the wall of the aneurysm (24-26). The inflammatory cells lead to activation of metalloproteinases. These processes lead to decrease in thickness of the aneurysm wall under the clot, which is favorable for its rupture (27). There are some publications, which suggest increasing risk of the aneurysm rupture near dynamically developing clot (28). However, there are no explicit evidence, which would justify change in management due to established presence of the clot.
Risk factors for the aneurysm occurrence
Analysis of risk factors for the aneurysm development is important especially in case of qualifying the patient for conservative treatment in case of small aneurysm. Limitation of risk factors may slow down the rate of dilation of the aorta.
Hypertension. It is the basic factor for incidence and rupture of the aneurysm (29). The aneurysm is diagnosed in 11.9% of patients with hypertension comparing to 6.5% of persons with normal blood pressure (30). Mechanism of occurrence of the abdominal aortic aneurysm in case of hypertension is not clearly explained, but it may be related to increased transmural pressure and overlapping the pulse wave reflected from the iliac arteries.
Smoking. Chronic smoking of cigarettes is possibly one of the most important environmental risk factors for occurrence of the abdominal aortic aneurysm (31). There is four-fold increase in risk of aneurysm occurrence in smoking persons comparing to non-smokers (32-34). The aortic aneurysm is diagnosed 7.6 times more frequently in current smokers and 3 times more frequently in former smokers than it is in non-smokers35. In reference to other locations of the vascular system diseases, smoking is associated with three-fold higher risk of occurrence of the abdominal aortic aneurysm comparing to occurrence of the coronary heart disease, and five-fold higher risk of this disease comparing to vascular disease of the central nervous system (36). Also the aneurysm growth rate in smokers is higher than it is in non-smokers, i.e. 2.83 mm/year and 2.53 mm/year, respectively (37). Products from the tobacco coming into the blood stream probably activate alpha 1-antitrypsin by melatonin oxidation, which leads to enzymatic damage of the aortic wall.
Age. Incidence of the abdominal aortic aneurysm increases with age. Prevalence of the aneurysm in the age of 65-69 years is 4.8%, and in the age of 80-83 years it is 10.8% (38). Together with aging, contamination of elastin with protein fractions increases, which results in calcification and defragmentation of its fibers. Weakening, i.e. loss of elasticity of the aortic wall during ageing process, is related to disintegration of the elastin fibers. Also phenomenon of apoptosis of the smooth muscle cells in the aortic wall is important in the aneurysm development, because it leads to reduction of their number, which results in intensification of the disintegration processes in the aortic wall (39).
Atherosclerosis. as the risk factor of the aneurysm. It is believed that atherosclerosis and hyperlipidemia (40) increase the risk of the aneurysm occurrence, but its mechanism is not known. Probably, the atherosclerotic deposits lead to weakening of the aortic wall, and in case of their regression, favorable conditions occur for the aneurysm development. Experimental animal studies demonstrated that treatment of hypercholesterolemia increases risk of the aneurysm occurrence (41). This phenomenon has not been confirmed in humans. Some studies do not show relationship or show insignificant relationship between decreasing rate of the aneurysm growth and administration of statins (42). In humans, the atherosclerotic plaques probably impair nutrition supply to the aortic wall provided by diffusion, which may weaken the tunica media. However, coexisting atherosclerosis and the aneurysm is probably caused by different causes and they are independent from each other.
Gender. Prevalence of the aneurysms increases in males after the age of 55 and in females after the age of 70 (43).
Chronic obstructive pulmonary disease (44). It may favor development of the aneurysm in mechanism of deficit of alpha 1-antitrypsin, which is present in both diseases. However, there is still no explicit evidence of faster development of the aneurysm in persons with COPD (45).
Risk factors for the aneurysm rupture
The aneurysm size is independent and the most important risk factor for the aneurysm rupture (46, 47).
There are the following factors, which are favorable for the aneurysm rupture: smoking, female gender, hypertension (48).
Some studies indicate five-fold higher risk of the aneurysm rupture in females than it is in males (49), which suggests use of the surgical treatment in case of smaller aneurysm’s size than 55 mm.
Relationship between the aneurysm size and risk of its rupture within the year is presented in table 1.
Table 1. Relationship between the aneurysm size and risk of ruplture.
Depending on the aneurysm size, the patient is qualified for conservative treatment and observation or for surgical treatment. Scheme of qualification is presented in figure 2.
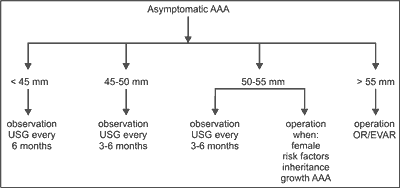 Fig. 2. AAA – scheme of procedure.
Indication for surgical treatment results from obtaining such size of the aneurysm that the risk of rupture exceeds the risk of surgery. UKSAT study, after analyzing 1090 cases with small aneurysm measuring 40-55 mm in diameter, showed no difference in 5-year mortality in the patients undergoing classic surgery comparing to the patients under observation (50). Risk of rupture was 1%, and mortality in case of surgical procedure was 5.6%. In ADAM study, these values were 0.6% and 2.7%, respectively. The males are qualified for the surgery if diameter of the aneurysm is at least 55 mm, and females, due to higher risk of rupture, are qualified for the surgery if diameter of the aneurysm is at least 52 mm (51).
Indications for urgent aneurysm repair are as follows:
1. Growth of the aneurysm diameter by 10 mm per year or 5 mm over 6 months.
2. Symptomatic aneurysm.
3. Ruptured aneurysm.
The following symptoms of the aneurysm advance decision on surgery:
1. Peripheral embolism (e.g. blue toe syndrome)
2. Acute thrombus formation
3. Back pain
4. Non-specific abdominal pain
5. Hydronephrosis
Limitations of the classic laparotomy method
According to UKSAT study, perioperative mortality is 5.6%, and according to ADAM study, 2.7%.
According to the study conducted by Hertzer et al., increase in mortality is connected with performing surgical treatment in patients in the age of over 75 years (52).
Classic method also brings the risk of not only systemic complications such as myocardial infarction, respiratory failure, renal failure, and stroke, but also local complications such as prosthesis inflammation, infection.
Perioperative complications in case of open laparotomy method are presented in table 2 (52).
Table 2. Perioperative complications OR.
30-day perioperative mortality may be reduced to 2% if the persons with heart diseases, respiratory system diseases and kidney diseases are disqualified from the classic treatment (53).
The persons, for whom the classic surgery is associated with increased risk should be qualified for endovascular treatment – EVAR
Endovascular repair of the abdominal aortic aneurysms – EVAR (Endovascular aneurysm repair).
There is two- to three-fold decrease in 30-day postoperative mortality in case of an elective intravascular procedure comparing to a classic surgery (1.2-1.6% vs. 4.6-6%) (54, 55).
EVAR comparing to classic surgery is minimally invasive procedure including endovascular insertion of a special prosthesis (stent graft) into the aneurysm cavity through the femoral artery approach. It is done in order to eliminate the aneurysm cavity from circulation. The procedure is usually conducted under epidural or local anesthesia (56). The benefits brought by EVAR include shortening the procedure time, limitation in number of general anesthesia, reduction in perioperative injury, reduction in postoperative pain, reduction of blood loss, significant limitation in necessity to stay at the intensive care unit and, which is the most important, reduction of perioperative mortality.
In order to place the stent graft correctly in the aneurysm, and reduce the risk of perioperative complications, suitable anatomical conditions have to be met. Size of the aneurysm as well as the subrenal segment of the aorta and diameters of the iliac arteries are presented in figure 3 and in table 3.
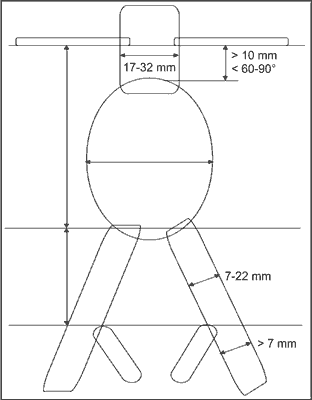 Fig. 3. The required measurements.
Table 3. EVAR – Limits dimensions.
Endovascular prosthesis is usually made of metal support structure in form of a large stent, which is covered with thin wall prosthesis made of Dacron or PTFE. The stent graft usually has the shape of bifurcated prosthesis, which comprise the subrenal tube and iliac branches. It is placed with the catheters through the femoral artery approach. The procedure starts from implantation of the main segment of the prosthesis going from the aorta to one of the iliac arteries, and then the procedure continues in implantation of the remaining segment going from contralateral side at the extension of the prosthesis going to the second iliac artery.
In order to correctly select the size of the prosthesis taking aneurysm anatomy into consideration, it is necessary to conduct precise measurement of the aneurysm, aorta and iliac arteries. The measurement is usually conducted by analysis of the computerized tomography imaging with contrast medium (57). The magnetic resonance imaging is a very specific evaluation for diagnosing the aneurysm, and it also may provide measurements, but due to its high cost and limited availability it is rarely performed.
Tomography is evaluated by reviewing slice by slice, as well as by building 3D reconstruction. Tomography should also be reviewed without administered contrast medium in order to examine atherosclerotic lesions of the neck, which should not exceed 50%, and the atherosclerosis of the iliac arteries, whether insertion of the system is possible. Depending on the manufacturer’s recommendations for a given prosthesis, the measurements are usually conducted within the lumen of the vessels or together with the wall thickness.
Figure 4 presents example of the dimensioning of AAA before EVAR.
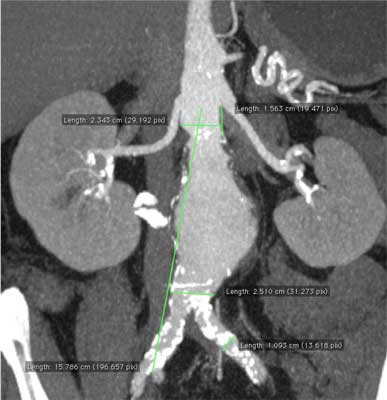 Fig. 4. Dimensioning of AAA in the computerized tomography angiogram.
Stent grafts differ from each other not only in structure of the carrier stent – which may be made of nitinol or steel, and in the prosthesis – which may be made of PTFE or Dacron, but also they differ in structure of fixing segment located at the level of the renal arteries. Depending on this segment, the prostheses are classified as subrenal and suprarenal. In each of these systems, covered part of the prosthesis begins below the renal arteries. In case of subrenal systems, the carrier stent also ends at the same level. In case of suprarenal systems, free, uncovered segment of the carrier stent protrudes over the level of the prosthesis and creates so-called crown of the stent graft. This crown may include additional fixing elements such as small hooks, which lock into the vessel wall after implantation, which additionally fixes the prosthesis.
After dimensioning AAA, the prosthesis is selected based on tables provided with the stent graft by the manufacturer.
Records reveal that within six-year observation there are no differences in late complications among various endovascular prostheses used in the procedures. Also, no differences were observed in terms of migration, bending, and occlusion of the prosthesis as well as frequency of repeated interventions and conversions (58).
Limitations of EVAR result from difficult anatomy of the aneurysm. Previously presented conditions should be met in order to make the graft tightly fit to the vessel walls and prevent leakage of the blood into the aneurysm cavity. The neck of AAA is very important. In case of short and wide neck, there is an increased risk that the prosthesis will slide off or will not be tight. In such cases, at the beginning of implantation, the attempt must be made to straighten the neck on the catheters or repair it with a rigid stent. In case of aneurysms reaching the renal arteries, the special prostheses are used, so-called fenestrated stent grafts, which have side holes for renal stents (59). In case of trapezoid shape of the neck directed towards the aneurysm cavity, either stent graft with planned overdimensioning of more than 20% should be considered or the fenestrated stent graft should be used – however, it should be emphasized that cost of such prosthesis is two/three times higher.
EVAR is associated with lower perioperative mortality comparing to open laparotomy method, but in long-term is associated with higher percentage of repeated interventions than the classic method (60).
Basic complication following EVAR is leakiness, which results in filling the aneurysm cavity with blood despite placement of the stent graft – there are so-called leakages.
Example of the stent graft placed into AAA is presented in figure 5.
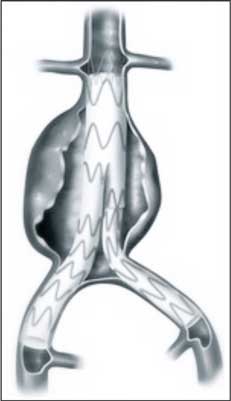 Fig. 5. Example of the stent graft placed into AAA.
Types of endoleakages are presented in table 4.
Table 4. Type of endoleakages.
Frequency of leakages within at least 30 days are presented in the table 5 (61, 62).
Table 5. Frequency of endoleakages in the time.
Type I of the endoleakages leads to increased pressure in the aneurysm cavity and it may cause its rupture. It requires repair. If it is caused by insufficient adhesion, repeated dilatation may be done or implantation of Palmaz stent, or if it is caused by migration, repeated implantation of the aortic or iliac extension should be done. Also, coiling of the leakage site may be performed. Finally, in order to repair the leakage, conversion to the classic method may be necessary (63).
Type II of the leakage is usually associated with low-pressure because of the lumbar or the visceral arteries naturally approaching the aneurysm cavity. Sometime, it is difficult to detect. It is established in 20% of cases within postoperative period. In 50-80% of cases, it spontaneously recedes within 6 months following the procedure (64). It requires repair, if it leads to increase in the aneurysm diameter (65). In such cases, e.g. coiling with microcatheters through the visceral branches is performed or laparoscopic repair of collaterals is done.
Type III of the leakage is treated by endovascular implantation of the covered stents.
Other causes of repeated interventions (late complications) are presented in table 6.
Table 6. Late complications of EVAR.
Comparison of the perioperative mortality and one- and five-year survival in EVAR and in the classic method is presented in table 7 (66).
Table 7. Comparsion of the perioperative mortality and one and five years survival EVAR/OR.
Discussion
Currently, both methods of AAA repair, classic and endovascular, are available for the patients. Efficacy of both methods is confirmed by clinical studies (67). Gradual increase in incidence of AAA, which is obviously connected with the extended life expectancy in population, intensity of risk factors and higher availability of imaging evaluations in diagnostics. Qualification for the surgical treatment has to consider safety of the patient, so the risk of surgery cannot exceed the risk related to the aneurysm itself. Males are qualified for the surgical treatment in case of stable aneurysm measuring at least 55 mm in diameter, and females in case of the aneurysm measuring at least 50 mm in diameter. The persons, for whom the risk of the classic procedure is too high, currently have the opportunity to undergo endovascular procedure. The first such procedure of implantation of the prosthesis through the inguinal approach was performed by Parodi in 1990 (68). Since that time, a constant dynamic development of technology has taken place, and it was difficult to compare use of these two systems at the beginning and for a long time. Currently, the studies and records are available, which support relatively objective comparison of both methods. In long-term observation, both methods provide comparable percentage of survival. Higher number of repeated interventions related to dysfunction of the implanted prosthesis is recorded in case of EVAR. Endovascular method is associated with lower perioperative mortality in patients, and it also provides opportunity of faster convalescence and early return to every day routine. Cost of the endovascular method, due to the price of the stent graft, is higher than it is in case of classic method, but it also depends on analysis of data of a given centers.
Currently, modification of the endovascular systems relates to obtaining as small diameters of the introduction systems as possible, which supports performing the procedure only with percutaneous method by puncturing instead of preparing the vessel, which may limit even more local complications and shorten hospitalization period.
Conclusions
Endovascular method of the aneurysm repair becomes the method of choice. There is increasing percentage of aneurysms detected in elderly persons, burdened with other diseases, for whom the classic method is associated with high risk. The procedure itself is minimally invasive, and the patient does not require staying at the intensive care unit, and convalescence period is significantly shorter than it is in classic method. Even if EVAR is associated with higher rates of late repeated interventions, it is more willingly accepted by the patients. Piśmiennictwo
1. Thompson MM: Controlling the expansion of abdominal aortic aneurysms. Br J Surg 2003; 90: 897-98.
2. Lindholt JS, Vammen S, Juul S et al.:The validity of ultrasonographic scanning as a screening method for abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 1999; 17: 472-5.
3. Ellis M, Powell JT, Greenhalgh RM: Limitations of ultrasonog- raphy for the surveillance of abdominal aortic aneurysms. Br J Surg 1991;78: 614-6.
4. US Preventive Services Task Force. Screening for abdominal aortic aneurysm. Ann Intern Med 2005; 142: 198-202.
5. The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aorticaneurysms. Lancet 1998; 352: 1649-55.
6. Johnston KW, Rutherford RB, Tilson MD et al.: Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991; 13: 452-58.
7. Szmidt J: Tętniaki. [W:] Podstawy chirurgii. Kraków, Medycyna Praktyczna, 2003; 947-962.
8. Gillum RF: Epidemiology of aortic aneurysm in the United States. J Clin Epidemiol 1995; 48: 1289-98.
9. Singh K, Bonaa KH, Jacobsen BK et al.: Prevalence and risk factors for abdominal aortic aneurysms in a population-based study:the Tromso Study. Am J Epidemiol 2001; 154: 236-44.
10. McGregor JC, Pollock JG, Anton HC: The value of ultrasonography in the diagnosis of abdominal aortic aneurysm. Scott Med J 1975; 20: 133-37.
11. Cronenwett JL, Krupski WC, Rutherford RB: Abdominal aortic and iliac aneurysm. Rutherford R.B. (red): Vascular Surgery. Saunders WB 2000; 20: 183-189.
12. Wanhainen A, Thermudo R, Ahlstrom H et al.: Thoracic and abdominal aortic dimension in70-years old men and women a population-based whole-body MRI study. J Vasc Surg 2008; 47: 504-12.
13. Gloviczki P et al.: Multiple aortic aneurysms: the results of surgical management. J Vasc Surg 1990; 11: 19.
14. Newman AB, Arnold AM, Burke GL et al.: Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: The Cardiovascular Health Study. Ann Intern Med 2001; 134: 182-190.
15. Heller JA, Weinberg A, Arnos R et al.: Two decades of abdominal aortic aneurysm repair: have we made any progress? J Vasc Surg 2000; 32: 1091-110.
16. Gomes MN, Davros WJ, Zemen RK: Preoperative assessment of abdominal aortic aneurysm. The value of helical and three – dimensional computed tomography. J Vasc Surg 1994; 20: 367-376.
17. Kent KC, Zwolak RM, Jaff MR et al.: Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg 2004; 39: 267-269.
18. Petersen MJ, Cambria RP, Kaufman JA et al.: Magnetic resonance angiography in the preoperative evaluation of abdominal aortic aneurysms. J Vasc Surg 1995; 21: 891-895.
19. Melton LJ, Birckerstaff LK, Hollier LH et al.: Changing incidence of abdominal aortic aneurysms – a population based study. Am J Epidemiology 1984; 120: 379-386.
20. Bergquist D: Abdominal aortic aneurysms. Eur Heart J 1997; 18: 545-546.
21. Lederle FA, Johnson G.R, Wilson SE et al.: Prevalence and associations of abdominal aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann. Intern. Med. 1997; 126: 441-449.
22. Scott RAP, Bridgewater SG, Ashton HA: The Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360: 1531-1539.
23. Mower WR, Quinones WJ, Gambhir SS: Effect of intraluminal thrombus on abdominal aortic aneurysm wall stress. J Vasc Surg 1997; 26: 602-8.
24. Vorp DA, Mandarino WA, Webster MW, Gorcsan J: Potential influence of intraluminal thrombus on abdominal aortic aneurysm as assessed by a new non-invasive method. Cardiovasc Surg 1996; 4: 732-9.
25. Vorp DA, Wang DH, Webster MW, Federspiel WJ: Effect of intraluminal thrombus thickness and bulge diameter on the oxygen diffusion in abdominal aortic aneurysm. J Biomech Eng 1998; 120: 579-83.
26. Vorp DA, Lee PC, Wang DH et al.: Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg 2001; 34: 291-9.
27. Di Martino E, Mantero S, Inzoli F et al.: Biomechanics of abdominal aortic aneurysm in the presence of endoluminal thrombus: experimental characterisation and structural static computational analysis. Eur J Vasc Endovasc Surg 1998;15:290-9.
28. Stenbaek J, Kalin B, Swedenborg J: Growth of thrombus maybe a better predictor of rupture et han diameter in patients with abdominal aortic aneurysms. EurJVascEndovascSurg 2000; 20: 466-9.
29. Powell JT, Greenhalgh RM: Small abdominal aortic aneurysms. N Engl J Med 2003; 348: 1895-1901.
30. Naydeck BL, Sutton-Tyrrell K, Schiller KD et al.: Prevalence and risk factors for abdominal aortic aneurysms in older adults with and without isolated systolic hypertension. Am J cardiol 1999; 83: 759-764.
31. Lee AJ, Fowkes FG, Carson MN et al.: Smoking, atherosclerosis and risk of abdominal aortic aneurysm. Eur Heart J 1997; 18: 671-676.
32. Vardulaki KA, Walker NM, Day NE et al.: Quantifying the risks of hypertension, age, sex and smoking in patients with abdominal aortic aneurysm. Br J Surg 2000; 87: 195-200.
33. Blanchard JF, Armenian HK, Friesen PP: Risk factors for abdominal aortic aneurysm: results of a case-control study. Am J Epidemiol 2000; 151: 575-83.
34. Lindholt JS, Heegaard NH, Vammen S et al.: Smoking, but not lipids, lipoprotein(a) and antibodies against oxidised LDL, is correlated to the expansion of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2001; 21: 51-56.
35. Wilmink TB, Quick CR, Day NE: The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg 1999; 30: 1099-1105.
36. Lederle FA, Nelson DB, Joseph AM: Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg 2003; 38: 329-34.
37. Brady AR, Thompson SG, Fowkes FG et al.: Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004; 110: 16-21.
38. Jamrozik K, Norman PE, Specer CA et al.: Screening for abdominal aortic aneurysm, lesions from a population-based study. Med J Aust 2000; 173: 345-350.
39. Li PF, Dietz R, Von Harsdorf R: Reactive oxygen species induce apoptosis of vascular smooth muscule cell. FeBS Lett 1997; 12: 602-603.
40. Blanchard AF, Armenian HK, Friesen PP: Risk factors for abdominal aortic aneurysm: results of a case-control study. J Epidemiol 2000; 151: 575-583.
41. Zarins CK, Glagov S, Vesselinovitch D, Wissler RW: Aneurysm formation in experimental atherosclerosis: relationship to plaque evolution. J Vasc Surg 1990; 12: 246-256.
42. Ferguson CD, Clancy P, Bourke B et al.: Association of statin prescription with small abdominal aortic aneurysm progression. Am Heart J 2010; 159: 307-13.
43. Rodin UB, Daviglus ML, Wong GC et al.: Middle age cardiovascular risk factors and abdominal aortic aneurysm in older age. Hypertension 2003; 42: 61-68.
44. Van Laarhoven CJHM, Borstlap AC, Van Berge-Henegouwen DP et al.: Chronic obstructive pulmonary disease and abdominal aortic aneurysm. Eur J Vasc Surg 1993; 7: 386-390.
45. Spencer C, Jamrozik K, Kelly S et al.: Is there an association between chronic lung disease and abdominal aortic aneurysm expansion? ANZ J Surg 2003.
46. Lederle FA, Johnson GR, Wilson SE et al.: Rupture rate of large abdominal aortic aneu- rysms in patients refusing or unfit for elective repair. JAMA 2002; 287: 2968-72
47. Norman PE, Powell JT: Abdominal aortic aneurysm: the prog- nosis in women is worse than in men. Circulation 2007;115: 2865-9.
48. Dalman RL, Tedesco MM, Myers J, Taylor CA: AAA disease: mechanism, stratification, and treatment. Ann NY Acad Sci 2006; 1085: 92-109.
49. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 2002; 346: 1445-52.
50. Powell JT, Brown LC, Forbes JF et al.: Final 12-year follow-up of surgery versus surveillance in the uk small aneurysm trial. Br J Surg 2007; 94: 702-8.
51. Forbes TL, Lawlor DK, DeRose G, Harris KA: Gender differences in relative dilatation of abdominal aortic aneurysms. Ann Vasc Surg 2006; 20: 564-8.
52. Hertzer NR, Mascha EJ, Karafa MT et al.: Open infrarenal abdominal aortic aneurysm repair: the Cleveland Clinic experience from 1989 to 1998. J Vasc Surg 2002; 35: 1145-54.
53. Elkouri S, Gloviczki P, McKusick MA et al.: Perioperative complications and early outcome after endovascular and open surgical repair of abdominal aortic aneurysms. J Vasc Surg 2004; 39: 497-505.
54. Greenhalgh RM, Brown LC, Kwong GP et al.: Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR Trial 1), 30 day operative mortality results: randomised control trial. Lancet 2004; 364: 843-8.
55. Prinssen M, Verhoeven EL, Buth J et al.: A randomised trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004; 351: 1607-18.
56. Bettex DA, Lachat M, Pfammatter T et al.: To compare general, epidural and local anaesthesia for endovascular aneurysm repair (EVAR). Eur JVasc Endovasc Surg 2001; 21(2): 179-84.
57. Zoetelief J, Geleijns J: Patient doses in spiral CT. Br J Radiol 1998; 71(846): 584-6.
58. Van Marrewijk CJ, Leurs LJ, Vallabhaneni SR et al.: Risk-adjusted outcome analysis of endo- vascular abdominal aortic aneurysm repair in a large population: how do stent-graft compare? J Endovasc Ther 2005; 12: 417-29.
59. Gustavo S. Oderich. Diameter-Reducing Wire to Facilitate Deployment of a Modi?ed Zenith Fenestrated Stent Graft. Ann Vasc Surg 2010; 24: 980-984.
60. The United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. New Engl J Med 2010; 362: 1863-71.
61. Ruppert V, Leurs LJ, Hobo R et al.: Tube stent-grafts for infrarenal aortic aneurysm: a matched-paired analysis based on EUROSTAR data. Cardiovasc Intervent Radiol 2007; 30: 611-618.
62. Biebl M, Hakaim AG, Oldenburg WA et al.: Midterm results of a single-center experience with commercially available devices for endovascular aneurysm repair. Mt. Sinai J Med 2005; 72: 127-135.
63. Kelso RL, Lyden SP, Butler B et al.: Late conversion of aortic stent grafts. J Vasc Surg 2009; 49: 589-95.
64. Maldonado TS, Rosen RJ, Rockman CB et al.: Initial successful manage- ment of type I endoleak after endovascular aortic aneurysm repair with n-butyl cyanoacrylate adhesive. J Vasc Surg 2003; 38: 664-70.
65. Jones JE, Atkins MD, Brewster DC et al.: Persistent type II endoleak after endo- vascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg 2007; 46: 1-8.
66. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005; 365: 2179-2186.
67. Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomized controlled trial. Lancet 2005; 365: 2187-2192.
68. Parodi JC, Palmaz JC, Barone HD: Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991; 5: 491-6.
otrzymano/received: 2012-05-14 zaakceptowano/accepted: 2012-06-11 Adres/address: *Włodzimierz Hendiger Department of Vascular Surgery and Angiology Medical Centre for Postgraduate Education The Jerzy Popiełuszko Memorial Bielański Hospital ul. Cegłowska 80, 01-809 Warszawa tel.: +48 (22) 569-02-85 e-mail: hendiger@interia.pl Artykuł Możliwości i ograniczenia w endowaskularnym zaopatrzeniu tętniaka aorty brzusznej w Czytelni Medycznej Borgis. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








