|
© Borgis - Postępy Nauk Medycznych 7, s. 595-598
Adrianna Łoniewska-Lwowska, *Jadwiga Fabijańska-Mitek, Katarzyna Koza
Dlaczego warto badać proteom krwinek czerwonych przechowywanych w bankach krwi
Why study proteome of red blood cells stored in blood banks**
Department of Immunohaematology, Medical Centre of Postgraduate Education, Warsaw
Head of Department: Jadwiga Fabijańska-Mitek,PhD Streszczenie
Zadaniem banku krwi jest takie przygotowanie krwinek czerwonych, by przenosiły wydajnie tlen do tkanek oraz zachowały długo żywotność i funkcje w krążeniu biorców. Odkrycie antykoagulantów oraz nowoczesnych roztworów konserwujących umożliwiło przechowywanie krwinek czerwonych do 42 dni. Transfuzjologia jest dziedziną medycyny o restrykcyjnym systemie kontroli jakości, którego usprawnianie wymaga między innymi zrozumienia zmian zachodzących w krwinkach czerwonych w trakcie ich przechowywania. Konieczność takich analiz wspierają klinicyści, najczęściej z oddziałów kardiologicznych, którzy wskazują na istnienie ryzyka związanego z przetaczaniem jednostek krwi długo przechowywanych oraz zawierających leukocyty. Do tej pory nie poznano procesów molekularnych leżących u podstawy starzenia się krwinek czerwonych i ich wpływu na efektywność transfuzji. Analiza proteomiczna wykorzystująca nowoczesne techniki spektrometrii masowej może w przyszłości służyć jako narzędzie oceny zmian zachodzących w trakcie przechowywania krwi. Może być pomocna w ulepszaniu procedur preparatyki i przechowywania krwinek czerwonych, zwiększając jakość i bezpieczeństwo transfuzji. Słowa kluczowe: proteom krwinek czerwonych, starzenie krwinek czerwonych, spektrometria masowa
Summary
The primary goal of any blood bank is to prepare red blood cells (RBCs) being able to efficiently deliver oxygen to the tissue and survive in the recipient circulation long enough to perform its functions. Discovery of blood anticoagulant and modern preservation solutions enabled to store the RBCs for 42 days. Transfusion medicine is characterized by very restrictive control system, which in order to be constantly improving, needs better understanding of RBC senescence processes undergoing during storage. The necessity of this analysis is supported by many clinicians, mostly from the cardiology departments, who point out different recoveries depending on the storage period and presence of leukocytes in the blood the patient received. However, till now the molecular bases of RBC senescence and its influence on transfusion efficiency are still not well defined. Proteomic analysis with accompany of modern mass spectrometry techniques reveals not only unexpected complexity of RBC proteome but also serves as a future tool to assess the changes that RBCs undergo during storage. This can be of immense help for further improvement of red blood cells preparation and storage methods, thereby increasing the quality and safety of transfusions. Key words: proteome of red blood cells, mass spectrometry, senescence of red blood cells
Introduction
The discovery in the 1915 of sodium citrate as blood anticoagulant (1) has started the possibility to store the blood in blood banks. Further introduction of modern preservation solutions as for example CPDA (citrate-phosphate-dextrose-adenine) or CPD+SAGM (saline-adenine-glucose-mannitol) used in Europe, prolonged the time of red blood cells (RBCs) storage to 42 days. The primary goal of every blood bank is to prepare RBCs being able to efficiently deliver oxygen to the tissue and survive in the recipient circulation long enough to perform its functions. The collection, processing, testing, production and storage procedures in blood banks are closely regulated by special directives and guidelines (2, 3). Until now many biochemical processes of RBCs have been well described. More and more is known about the functions of individual molecules of erythrocyte membrane. But still little understood is the total protein composition of erythrocytes, nor the changes that RBC undergo during 120 days of their life, in conditions of many hematological disorders and during storage under blood bank conditions.
RBC storage lesions
During storage RBCs undergo a number of biochemical, morphological and metabolic changes known as “storage lesions”. For example, it is well described, that due to the accumulation of lactic acid in the blood bag, the pH decreases, and this increases phosphatase 3 enzyme activity, which results in 2,3-diphosphoglycerol (2,3-DPG) degradation. Low level of 2,3-DPG results in increased oxygen affinity to hemoglobin, and thereby causes diminished delivery of oxygen to tissues (4). During RBCs storage period there appear also changes in ATP level what can reflect on reology of erythrocyte (5), loss of S-nitrosothiol-haemoglobin (SNO-Hb) – and thereby decreased NO production and further vasodilatation, as well as membranous band 3 protein and some other proteins rearrangements (6). RBCs display unique reological possibilities provided, among other, by the sophisticated interactions of membrane and cytoskeletal proteins. This enables passage of RBCs through the blood vessels, which are often narrower than erythrocytes. Reduced flexibility will result in impaired perfusion and oxygen delivery to peripheral tissues; moreover, rigid RBCs might directly block capillaries. There are evidences from the in vitro analysis that RBCs storage induces a broad range of impairments of RBC hemodynamic behavior such as deformability, aggregability, and adherence to endothelial cells (7). It was also reported that serious hemorheological disorders, including the decrease in RBC deformability secondary to shape abnormalities, acidosis, and the decrease of blood clotting, start already at the second week of storage and progress up to the end of the storage period (8). There are also evidences of leukocyte influence on increase of RBCs adherence to the endothelial cell layer (9). In the membrane are localized inhibitors of complement pathway, protecting from haemolysis (10) and also proteins taking part in clotting process, transporters, receptors, adhesion molecules and molecules carrying RBC antigens (11).
Red blood cells are the most commonly transfused blood component. Some of the “storage lesions” mentioned above lead to removal of erythrocytes from the circulation but still a huge percentage of RBCs is recovered after transfusion and it is assessed that around 70-75% of them are present in the circulation after 24 hours post transfusion (12).
Adverse results of transfusions of stored RBCs
For twenty years now, there is alive debate concerning the functionality of fresh, several days stored, versus old – 14, 21, 28 or 35 ≥ days long stored blood, as well as leucoreduced versus non-leucoreduced RBCs. Opinion that the functionality of transfused blood from different storage periods can not be regarded as a comparable is supported by many clinicians, mainly from cardiology units who pay attention on different recoveries depending on the storage period and presence of leukocytes in the blood the patient received. These concerns mostly patients with acute coronary syndrome, ischemic heart disease or after coronary interventions, who are particularly susceptible to effects of hypoxia, activation of thrombosis, effects of heamolysis. Many authors demonstrated an increased in-hospital and out-of-hospital mortality among hospitalized patients associated with increased mean age of RBCs transfused (13, 14). Study of patients who were undergoing coronary-artery bypass grafting, cardiac-valve surgery, revealed that transfusion of RBCs stored for more than 2 weeks was associated with a significantly increased risk of postoperative complications. In-hospital death, prolonged intubation, renal failure, septicemia or sepsis, multiorgan failure, and a composite of serious complications were all more frequent in patients given blood stored for more than 14 days. Furthermore, survival, particularly in the first 6 months after surgery, was significantly reduced (15).
However, one should also take into account the possibility that there is no problem with the storage of RBCs, but rather an incorrect analysis of the cases examined (16).
In order to elucidate the concerns regarding the changes the blood products undergo, there is a need of global and more sophisticated analysis to be engaged. The proteomic analysis can be of immense help in this case.
Elements of proteomic analysis and present achievements in understanding of RBC proteome
So far much has been learned from classical biochemical approaches about RBC composition and physiology, but a new tool – mass spectrometry (MS) and proteomic analysis allow increasing our understanding of the RBC. The term “proteome”, referring to the protein product of the genome, was first coined in 1996 (17). The proteome is a complex and dynamic entity. Unlike the relatively static genome, the proteome is controlled by an astonishing number of factors. Proteomics is the analysis of the whole proteome of a tissue, organism or body fluid. The principle is based on the extraction of proteins, fractionation and purification of the extract, proteolytic digestion of proteins into peptides, final separation and detection by mass spectrometry. MS measures the mass-to-charge ratio of ionized protein sample. The final step is the protein sequences identification using databases. Mainly, two systems are used in protein/peptide fractionation: in-gel or gel-free. Two-dimensional gel electrophoresis (2DE) is performed following a first dimension of isoelectric focusing (IEF) (separation according to isoelectric point [pI]) and a second dimension of SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), where separation is performed according to apparent molecular weight. Afterwards, proteins are digested in-gel and peptides are analyzed by MS. The gel-free separation is based on liquid chromatography (LC). LC separation can be performed at the protein or the peptide level and directly coupled to a mass spectrometer for on-line analysis (LC-MS/MS). Next to the quality application, MS became quantitative, and both label-free and tagging methods are available for protein quantification. Due to the steady improvement, proteomic technology became widely applied in many medical disciplines.
Proteomics and red blood cells met a decade ago, with the need to map and understand the proteome of RBCs. The first study of the RBC membrane’s proteome was performed and published in 2002 (18). Low et al. focused on plasma membrane proteins and used the classical approach of IEF-SDS PAGE, followed by silver staining, in gel trypsin digestion and MALDI-TOF (matrix-assisted laser desorption/ionization-time-of-flight) mass spectrometry analysis. This approach allowed identifying 102 distinct RBC membrane proteins. Two years later, in 2004 the available list of the erythrocyte proteome constituted of the total number of 181 proteins (19). During the next 8 years, list of identified RBC proteins has extended by 10 times (fig. 1), nowadays there is 1578 of cytosolic (20) and 314 of membrane RBC protein being identified (21).
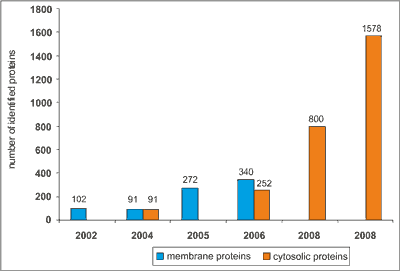 Fig. 1. Development of the proteomic analysis methods and its influence on the numbers of identified RBC proteins:
2002: 102 RBC membrane proteins identified by 1-DE, 2-DE (IEF-SDS), MALDI-TOF (18), 2004: 91 RBC membrane and 91 cytosolic proteins identified by μLC/MS-MS (19), 2005: 272 RBC membrane proteins identified by Proteolytic Digestion Chip and two-dimensional ESI-MS/MS (31), 2006: 340 RBC membrane and 252 cytosolic proteins identified by 1DE (SDS) in gel-digestion, LC-ESI-MS/MS (21), 2008: 800 proteins identified via aminoacid/peptide depletion method, 2D (IEF-SDS), nanoLC-ESI-MS/MS (33, 34), 2008: Ligand library”ProteoMiner”-2D (IEF-SDS), nanoLC-MS/MS-LQTOrbitrap (20). This was achieved mostly due to the application of different MS methodologies, as well as usage of sophisticated methods of protein depletion (e.g. ligand library) (22). However, till now there are only few proteomic publications addressing the changes that RBCs undergo during storage (23-27). Studies mentioned above were undertaken with the aim of describing the changes of the RBC cytoskeleton during storage of SAGM-preserved nonleukodepleted RBCs units, focusing on the influence of oxygen on the protein degradation process (25). Basing on the 2D electrophoresis followed by protein identification by ESI-MS/MS (electrospray ionization mass spectrometry) of RBCs samples stored with leucocytes in the presence of oxygen or/and protein inhibitors, D’Amici and coworkers concluded that cytoskeletal storage-dependent lesions come from oxidation rather than proteolysis (25). Anniss et al. analyzed the influence of leukoreduction on the number and types of proteins present in the supernatants of stored RBCs units (24). Researchers pointed out the role of leukocytes and platelets, which release proteins that may contribute to RBCs degradation.
Well described phenomenon of membrane blebbing and vesicles formation during in vivo RBC senescence (28, 29), forced other groups to focus their researches on proteins of vesicles accumulating in supernatants during blood storage. Rubin et al. observed the huge increase in microparticles (MPs) formation during erythrocyte concentrates (EC) storage, and theirs proteomic analysis revealed characteristic protein composition (27). Our knowledge according micro- and nanoparticles formation and RBC protein composition changes has been enriched by Bosman et al. (23), who suggest the very similar storage-changes in protein constitution of vesicles when compared with membrane of stored RBCs. Zolla’s group focused on the storage-dependent remodeling of erythroid membrane proteins upon aerobic versus anaerobic condition (26). Proteins characterized by its changing level upon storage time were determined using reducing and nonreducing SDS-PAGE and were identified by tandem mass spectrometry. Obtained results indicate the atypical presence of cytoplasmic proteins – i.e. peroxiredoxin 2 (Prx-2) in membranous fraction of stored RBCs. Prx-2 was thereby proposed as a biomarker of oxidative damage or aging status.
Researches cited above concerning changes that RBCs undergo during storage in blood bank conditions have provided a lot of very important and useful information, however still it is far to final conclusions, because of many technical problems.
The exquisite sensitivity of modern mass spectrometers requires pure population of cells, which means that contamination of the RBC preparation by even low levels of leukocytes or reticulocytes can be problematic. As far as separation of erythrocytes from white blood cells is done routinely in blood banks during preparation of leucodepleted RBCs, so far reticulocytes are always present in the blood units. It is known that during maturation reticulocytes loose up to 20% of surface area due to membrane vesiculation (30), a decrease in cell volume, an increase in membrane mechanical stability and acquisition of biconcave shape, implying a major reorganization of membrane and skeletal components. In recent years process of exosome release by reticulocytes has been extensively studied (31), pointing out that this process contributes to membrane loss and remodeling of its architecture. Therefore analysis of sample containing reticulocytes proteins may bring onto misleading conclusions. For proteomic analysis of stored RBC there is an urgent need to establish methods of isolation of pure, homogeny population of RBCs in order to perform perfect protein identification.
At the present, we have introduced a project concerning the analysis of proteome of stored RBC. Goals of our study are 1) to elaborate efficient isolation method of pure population of erythrocytes from stored RBCs, 2) MS analysis of RBC proteome changes during their storage under blood bank conditions, 3) to identify biomarker/s of RBC in vitro senescence that can be useful in future transfusiological practice.
**This study was supported by 501-1-26-02-12 grant. Piśmiennictwo
1. Zubair AC: Clinical impact of blood storage lesions. American Journal of Hematology 2010; 8: 117-122.
2. Seitz R, Heiden M, Nubling CM et al.: The harmonization of the regulation of blood products: a European perspective. Vox Sang 2008; 94: 267-276.
3. Łętowska M et al.: Medyczne zasady pobierania krwi, oddzielania jej składników i wydawania, obowiązujące w jednostkach organizacyjnych publicznej służby zdrowia. Warszawa, Instytut Hematologii i Transfuzjologii 2011; 1-480.
4. Högman CF: Preparation and preservation of red cells. Vox Sang 1998; 74 (2 Suppl): 177-187.
5. Greenwalt TJ, Bryan DJ, Dumaswala UJ: Erythrocyte membrane vesiculation and changes in membrane composition during storage in citrate-phosphate-dextrose-adenine-1. Vox Sang 1984; 47: 261-270.
6. Jia L, Bonaventura C, Bonaventura J, Stamler JS: S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 1996; 380: 221-226.
7. Relevy H, Koshkaryev A, Manny N et al.: Blood banking-induced alteration of red blood cell flow properties. Transfusion 2008; 48: 136-146.
8. Berezina TL, Zaets SB, Morgan C et al.: Influence of storage on red blood cell rheological properties. J Surg Res 2002; 102: 6-12.
9. Anniss AM, Sparrow RL: Storage duration and white blood cell content of red blood cell (RBC) products increases adhesion of stored RBCs to endothelium under flow conditions. Transfusion 2006; 46: 1561-1567.
10. Miwa T, Song WC: Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol 2001; 1: 445-459.
11. Mori D, Yano K, Tsubota K et al.: Computational study on effect of red blood cells on primary thrombus formation. Thromb Res 2008; 123: 114-121.
12. Lion N, Crettaz D, Rubin O, Tissot JD: Stored red blood cells: a changing universe waiting for its map(s). J Proteomics 2010; 73: 374-385.
13. Basran S, Frumento RJ, Cohen A et al.: The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesth Analg 2006; 103: 15-20.
14. Eikelboom JW, Cook RJ, Liu Y, Heddle NM: Duration of red cell storage before transfusion and in-hospital mortality. Am Heart J 2010; 159: 737-743.
15. Gorman Koch C, Li L, Sessler DI et al.: Duration of Red-Cell Storage and Complications after Cardiac Surgery. N Engl J Med 2008; 358: 1229-1239.
16. van de Watering L: Red cell storage and prognosis. Vox Sang 2011; 100: 36-45.
17. Wilkins MR, Sanchez JC, Gooley AA et al.: Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev 1996; 13: 19-50.
18. Low TY, Seow TK, Chung MCM: Separation of human erythrocyte membrane associated proteins with one-dimensional and two-dimensional gel electrophoresis followed by identification with matrixassisted laser desorption/ionization-time of flight mass spectrometry. Proteomics 2002; 2 (9): 1229-1239.
19. Kakhniashvili DG, Bulla Jr. LA, Goodman SR: The human erythrocyte proteome: Analysis by ion trap mass spectrometry. Molecular and Cellular Proteomics 2004; 3: 501-509.
20. Roux-Dalvai F, Gonzalez de Peredo A, Simó C et al.: Extensive analysis of the cytoplasmic proteome of human erythrocytes using the peptide ligand library technology and advanced mass spectrometry. Mol Cell Proteomics 2008; 7: 2254-2269.
21. Pasini EM, Kirkegaard M, Mortensen P et al.: In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood 2006; 108 (3): 791-801.
22. Righetti PG, Boschetti E, Lomas L, Citterio A: Protein Equalizer Technology: the quest for a “democratic proteome”. Proteomics 2006 Jul; 6 (14): 3980-3992.
23. Bosman GJ, Lasonder E, Luten M et al.: The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion 2008; 48: 827-835.
24. Annis AM, Glenister KM, Killian JJ et al.: Proteomic analysis of supernatants of stored red blood cell products. Transfusion 2005; 45: 1426-1433.
25. D’Amici GM, Rinalducci S, Zolla L: Proteomic analysis of RBC membrane protein degradation during blood storage. J Proteome Res 2007; 6: 3242-3255.
26. Rinalducci S, D’Amici GM, Blasi B et al.: Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion 2011; 51: 1439-1449.
27. Rubin O, Crettaz D, Canellini G et al.: Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang 2008; 95: 288-297.
28. Willekens FL: Roerdinkholder-Stoelwinder B, Groenen-Döpp YA et al.: Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood 2003; 101: 747-751.
29. Willekens FL, Werre JM, Kruijt JK et al.: Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood 2005; 105: 2141-2145.
30. Mohandas N, Groner W: Cell membrane and volume changes during red cell development and aging. Ann N Y Acad Sci 1989; 554: 217-224.
31. Blanc L, De Gassart A, Géminard C et al.: Exosome release by reticulocytes-an integral part of the red blood cell differentiation system. Blood Cells Mol Dis 2005; 35: 21-26.
32. Tyan YC, Jong SB, Liao JD et al.: Proteomic profiling of erythrocyte proteins by proteolytic digestion chip and identification using two-dimensional electrospray ionization tandem mass spectrometry. J Proteome Res 2005; 4 (3):748-757.
33. Bachi A, Simó C, Restuccia U et al.: Performance of combinatorial peptide libraries in capturing the low-abundance proteome of red blood cells. 2. Behavior of resins containing individual amino acids. Anal Chem 2008; 80: 3557-3565.
34. Simó C, Bachi A, Cattaneo A et al.: Performance of combinatorial peptide libraries in capturing the low-abundance proteome of red blood cells. 1. Behavior of mono- to hexapeptides. Anal Chem 2008; 80: 3547-3556.
otrzymano/received: 2012-05-07 zaakceptowano/accepted: 2012-06-04 Adres/address: *Jadwiga Fabijańska-Mitek Department of Immunohaematology, Medical Centre of Postgraduate Education, Warsaw ul. Marymoncka 99/103, 01-813 Warszawa tel.: +48 (22) 569-38-20 e-mail: biofizyka@cmkp.edu.pl Artykuł Dlaczego warto badać proteom krwinek czerwonych przechowywanych w bankach krwi w Czytelni Medycznej Borgis. |
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








