|
© Borgis - Postępy Nauk Medycznych 8, s. 587-594
*Jarosław Kozakowski, Wojciech Zgliczyński
Hypocaloric diet and pharmacological treatment reduces body weight, insulin resistance and androgen levels and restore menstruation in obese women with polycystic ovary syndrome
Wpływ niskokalorycznej diety i leczenia farmakologicznego na masę ciała, wrażliwość na insulinę, androgeny oraz na cykl miesiączkowy u otyłych kobiet z zespołem policystycznych jajników
Department of Endocrinology, Medical Center of Postgraduate Education, Bielański Hospital, Warsaw, Poland
Head of Department: prof. dr hab. med. Wojciech Zgliczyński Streszczenie
Wstęp: Celem pracy była ocena wpływu diety niskokalorycznej w połączeniu z leczeniem farmakologicznym (sibutramina) na ciężar i skład ciała, masę tłuszczu, szczególnie w obrębie tułowia, wrażliwość na insulinę, androgeny oraz miesiączkowanie u kobiet z zespołem policystycznych jajników (PCOS) z nadwagą i otyłością. Materiał i metody: 20 kobiet z PCOS w wieku 19-49 lat z BMI 27,3-53,8 kg/m2, które były badane przed oraz po 1, 3 i 6 miesiącach leczenia dietą niskokaloryczną i sibutraminą w dawce 10-15 mg/dobę. Wyniki: Ciężar ciała, BMI i obwód talii obniżyły się znamiennie już po pierwszym miesiącu leczenia (p<0,001) i ulegały dalszemu obniżaniu w okresie kolejnych miesięcy. W czasie 6 miesięcy masa tłuszczu spadła o 9,2% (p<0,001), a masa tłuszczu tułowia o 8,2% (p<0,001). Znamiennemu obniżeniu uległo stężenie w surowicy glukozy (p<0,01) i insuliny (p<0,01). Bardzo wyraźnie, choć nieznamiennie obniżył się wskaźnik HOMA. Znamiennie obniżyło się stężenie cholesterolu całkowitego (p<0,01), cholesterolu LDL (p<0,05) i triglicerydów (p<0,01) oraz wzrosło stężenie cholesterolu HDL (p<0,001). Już po pierwszym miesiącu leczenia obniżyło się znamiennie stężenie testosteronu (p<0,05), androstendionu (p<0,05) i DHEA-S (p<0,05). Stężenie androgenów obniżało się dalej w okresie kolejnych miesięcy. U osiemnastu kobiet powróciło miesiączkowanie. Wnioski: W trakcie stosowania diety niskokalorycznej i leczenia farmakologicznego za pomocą sibutraminy obserwowano istotne zmniejszenie ciężaru ciała, korzystne zmiany wskaźników metabolicznych i czynników ryzyka chorób sercowo-naczyniowych, obniżenie stężenia androgenów i powrót miesiączkowania u większości kobiet z nadwagą i otyłością chorujących na PCOS. Słowa kluczowe: otyłość, insulinooporność, androgeny, PCOS
Summary
Objective: To estimate the effects of the hypocaloric diet and pharmacological treatment with sibutramine on body weight, fat mass, especially abdominal fat, insulin resistance, serum androgens and menstruation pattern in overweight and obese women with polycystic ovary syndrome. Material and methods: 20 females with PCOS aged 19-49 years with BMI 27.3-53.8 kg/m2 studied before and after 1, 3 and 6 months of the treatment with hypocaloric diet and sibutramine 10-15 mg/day. Measurement included body weight, BMI, waist circumference, total and trunk fat mass, glucose, insulin, blood lipids, androgens, LH, FSH, estradiol, fT4, TSH and menstrual pattern. Results: Body weight, BMI and waist circumference significantly decreased already after 1st month (p<0.001) and were decreasing further during next months. Fat mass decreased of 9.2% (p<0.001) and fat mass of the trunk of 8.2% (p<0.001) after 6 months. In the same time significant decrease in serum fasting glucose (p<0.01), insulin (p<0.01), total cholesterol (p<0.01), LDL-cholesterol (p<0.05) and triglicerydes (p<0.01) were observed. HDL-cholesterol increased significantly (p<0.001). Throughout the study serum testosterone, androstendione and DHEA-S levels decreased significantly (p<0.01; p<0.05 and p<0.001, respectively). Menstrual bleedings reoccurred in eighteen women throughout the study. Conclusions: Hypocaloric diet plus pharmacological treatment with sibutramine caused a significant weight loss, had beneficial effects on some metabolic and cardiovascular risk factors, reduced androgens and restored menstruations in overweight and obese women with polycystic ovary syndrome. Key words: obesity, insulin resistance, androgens, PCOS
Introduction
Polycystic ovary syndrome (PCOS) is one of the most frequent endocrine disorders in women in the reproductive age. The overall prevalence of PCOS in this population is estimated to be 5-10% (1). In 2003 an international consensus group in Rotterdam recommended that the diagnostic criteria for PCOS include ovarian dysfunction evidenced by at least two of the following features: 1) oligo- or anovulation, 2) clinical and/or biochemical signs of hyperandrogenaemia and 3) polycystic ovaries in the absence of other conditions that can cause similar signs and symptoms (2). The pathogenesis of this disorder is still far from full elucidation and is considered to result of complex of genetic and environmental factors.
Women with PCOS may experience a wide variety of symptoms, which may change over time, although abnormal uterine bleeding, symptoms of androgen excess: acne or hirsutism and polycystic ovaries are common features. In laboratory data disturbed ovarian and adrenal steroidogenesis, reduced levels of SHBG, insulin resistance with subsequent hyperinsulinemia and unfavorable lipid profile can be found (3-5).
Approximately half of the women with this syndrome are overweight or obese (6). It was found that patients with PCOS present mostly central type of obesity, whereas 70% of lean women have rather an android distribution of fat (7). Abdominal obesity is a major underlying factor in insulin resistance. However, insulin resistance was found also in lean women with PCOS (8). Insulin resistance leads to elevated circulating glucose and increase pancreatic insulin secretion resulting in hyperinsulinemia, that may in turn contribute to hypertension. Associated defects include the proinflammatory state with increased circulating of interleukin-6, TNFα and other cytokines. Cytokines and fatty acid also increase the production of fibrinogen and PAI-1 by the liver causing a prothrombotic state (9). As a consequence patients with PCOS have unfavorable cardiovascular risk profile (10, 11). Gynoid fat is considered to be much less active in this matter and is in fact believed to protect against atherogenesis. In women with PCOS obesity is also associated with androgen excess additionally contributing to vascular damage and to fertility disorders (12).
Several approaches have been proposed to correct metabolic abnormalities, restore menstruations and to induce ovulations in PCOS patients. These methods usually include lifestyle changes early on such as diet and exercises and use of various ovulation-inducing agents (eg, clomiphene-citrate, aromatase inhibitors, gonadotropins), insulin-sensitizing drugs (eg, metformin), and surgical treatment (eg, laparoscopic ovarian drilling, laser surgery) (13-16). Large randomized trials that have been conducted to compare clomiphene with metformin as a first-line treatment for anovulatory infertility have given conflicting results, so there is no unambiguous view in this matter (13, 17). Because metabolic alterations resulting from excess weight, mostly in form of abdominal obesity are widely considered the pivotal feature of PCOS approach pointed at lowering of the body weight, especially reducing abdominal fat seems to be the causal treatment of choice. Although there is no doubting in efficacy of lifestyle modification it is extremely difficult to maintain weight loss in obese patients only on that way. More weight loss and better metabolic results have been found in patients who in addition have taken insulin-sensitizing (metformin) or weight-lowering (sibutramin, rimonabant) drugs (18-22).
The aim of our study was to estimate the effects of the hypocaloric diet and pharmacological treatment with sibutramine on body weight, fat mass, especially abdominal fat, insulin resistance, serum androgens and menstruation pattern in overweight and obese women with polycystic ovary syndrome.
Material and methods
A total of twenty females with PCOS aged 19-49 years, mean 30.2 ± 8.5 (x ± SD) were studied. Their BMI were 27.3-53.8 kg/m2, mean 38.1 ± 6.4. One women was overweight and nineteen were obese. The diagnosis of PCOS was based on mentioned above criteria of the Rotterdam consensus. Oligomenorrhea was defined as menstrual periods that occur at intervals of greater than 35 days, with only four to nine periods in a year, and amenorrhea as the complete absence of menstruation. Clinical hyperandrogenaemia was defined as presence of hirsutism or acne. Biochemical hyperandrogenaemia was defined as serum testosterone levels greater than 0.9 ng/ml, androstendione levels greater than 310 ng/dl and dehydroepiandrosterone-sulfate levels greater than 2000-4100 ng/ml, depending on age. Ovary in USG were defined as polycystic when they included either 10 or more follicles measuring 2-9 mm in diameter or their volume was greater than 10 cm3.
The exclusion criteria included hypothyroidism, hyperprolactinemia, Cushing's syndrome, nonclassical congenital adrenal hyperplasia, and current or previous (within the last 3 months) use of oral contraceptives and other hormonal, antidiabetic and antiobesity drugs.
Subjects were studied in the course of the four visits in clinic: before treatment and after one, three and six months of dietary and drug intervention. Each visit included full physical examination with measurement of waist circumference, body height and weight, and then body mass index (BMI) was calculated. Blood was collected after an overnight fast at about 0800 h for glucose, lipids (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglicerydes), insulin, LH, FSH, estradiol, testosterone, androstendione, dehydroepiandrosterone-sulfate, TSH and free tyroxine through an iv catheter placed in the forearm. To estimate insulin resistance the HOMA index was calculated by the formula: fasting plasma insulin (microinternational units per milliliter) x fasting plasma glucose (millimoles per liter)/22.4. Subjects were considered as insulin resistant when HOMA index was> 2.5. Hypercholesterolaemia was defined as total cholesterol level above 5.2 mmol/l and hypetrigicerydaemia when trigicerydes level was higher than 1.81 mmol/l. During the first visit all of the subjects underwent transvaginal ultrasonography (TV-USG) and USG of abdomen to exclude adrenal pathology. At the start and in the end of the study body composition by dual-energy X ray absorptiometry (DEXA) was determined. The same operator performed all DEXA measurements.
From the beginning of the study all patients were on hypocaloric diet, that was determined individually. In each case the mean caloricity of current diet was calculated, and then the new regimen, containing 700 kcal per day less compared with initial caloricity was compiled. While continuing dietary treatment, women were received sibutramine (sibutramini hydrochloride, Zelixa, Biofarm, Poland) in the initial dose 10 mg daily. This dose was increased to 15 mg per day when the decline of body weight was not satisfying, it means that was lower than 0.5-1 kg per week. In two cases dose was reduced after third month of the treatment. Two subjects with considerable insulin resistance were additionally treated with metformin 500 mg twice a day. Four patient were on stable treatment with l-tyroxine.
Before the start of the study all subject were required not to become pregnant because of the risk of sibutramine (and metformin) in case of pregnancy and instructed to use adequate birth control methods. The consent was obtained from all of the participants.
Assays
Glucose was measured with glucose hexokinase reagent set with sensitivity 2.16 mg/dL. An enzymatic colorimetric method was used to measure total cholesterol in the presence of cholesterol oxidase and esterase. The sensitivity was 0.116 mg/dL. HDL-cholesterol was measured with enzymatic colorimetric method, sensitivity was 3 mg/dL. Triglicerydes were also measured with enzymatic colorimetric method with sensitivity 0.85 mg/dL. All mentioned biochemical measurements were performed using Roche Cobas Integra 400 chemistry analyzer (Roche Diagnostics). Insulin was measured by immunoradiometric method (Insulin IRMA – Immunotech SA, France); sensitivity was 2.0 mIU/ml. LH, FSH, TSH and free thyroxin (fT4) were measured by immunochemiluminescence method with IMMULITE 2000 (Siemens Healthcare Diagnostics, Inc). Estradiol was measured with the same IMMULITE 2000 analyzer; sensitivity was 15 pg/ml. Total testosterone was measured by RIA-CT method (Immunotech SA, France); sensitivity of this method was 0.025 ng/ml. Androstendione was measured by direct RIA-CT (DSL, USA). Dehyrdroepiandrosterone-sulfate was measured by RIA-CT method (Spectria, Orion Diagnostica, Finland); sensitivity of this method was 10 ng/ml.
Body mass index was calculated as a body weight (kg)/height2 (m2). Subjects with BMI between 25 and 30 kg/m2 were considered as overweight, whereas subjects with BMI between 30 and 40 kg/m2 were considered as obese, and with BMI above 40 kg/m2 as morbidly obese.
To measurements of body composition by DEXA method we performed a total body scans with use of Lunar Prodigy (GE Lunar, Madison, WI, USA) equipment, that was calibrated each day with a standardized phantom and serviced regularly. The coefficient of variation for measurements of body composition with this method is about 2%.
Statistical analysis
All the data are presented as the mean ± SD. The normality of the distribution of variables were verified with a Kolmogorov-Smirnov and Lilieforse tests. To examine bivariate relationships between data Pearson correlation or Spearman rank analyses were used. Comparisons between groups with normal distribution of the data were performed by unpaired Student's t-test, in other cases comparisons were performed by Kolmogorov-Smirnov test for two samples. For all analysis, a two-tailed P = 0.05 was considered to indicate statistic significance.
Results
Twenty females participated in the study. Their mean age was 30.2 ± 8.6 years. In table 1 baseline anthropometric data, body mass index and fat, blood pressure and biochemical results of the studied subjects are shown. The cohort represented a relatively broad range of age. One patient was overweight, eleven were obese and eight were considered as morbidly obese. DEXA results showed that all of the subjects had increased abdominal fat. Two women were hypertensive. In eight of them hypercholesterolemia and in ten hypertrigicerydemia were found.
Table 1. Baseline anthropometric characteristics, blood pressure and biochemical data of the study subjects.
BMI, body mass index; WC, waist circumference; FM, fat mass; FMt, fat mass of the trunk; BP, blood pressure.
In table 2 initial hormonal results are shown. All women were hyperandrogenic and all except one were euthyroic. Seven had elevated fasting serum insulin levels, and fifteen were considered as insulin resistant according to HOMA index.
Table 2. Hormonal results at the beginning of the study.
HOMA, homeostatic model assessment; DHEA-S, dehydroepiandrosterone-sulfate; LH, luteinizing hormone; FSH, follicle stimulating hormone; TSH, thyroid stimulating hormone; fT4, free thyroxin.
The effects of diet plus sibutramine on fat parameters are shown in the table 3. In all subjects very significant decrease in all measured parameters was observed. Fat mass decreased of 9.2% while fat mass of the trunk decreased of 8.2% during the 6 months of the study.
Table 3. Body weight, waist circumference and fat mass before and after treatment.
aP<0.0001
BMI, body mass index; WC, waist circumference; FM, fat mass; FMt, fat mass of the trunk. Treatment had great impact on glucose metabolism indices and on lipid profile (see table 4). Significant decrease in serum fasting glucose and insulin levels were observed after 6 months of the treatment. HOMA index decreased nearly significantly in the same time. Total cholesterol, LDL-cholesterol and triglicerydes significantly decreased, while HDL-cholesterol significantly increased during treatment.
Table 4. Parameters related to hyperinsulinemia and blood lipids
aP<0.05, bP<0.01, cP<0.001
TC, total cholesterol; LDL, low-density cholesterol; HDL, high-density cholesterol; TG, triglicerydes. Figures 1, 2 and 3 shows significant decrease in serum levels of testosterone, androstendione and DHEA-S during 6 months of diet regimen and sibutramine treatment.
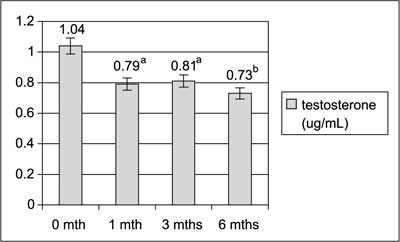 Fig. 1. Serum testosterone levels throughout the study.
aP<0.05 bP<0.01 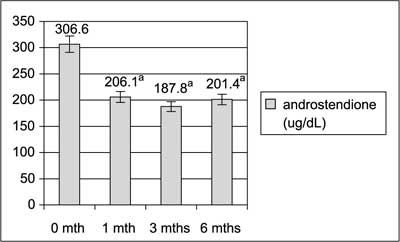 Fig. 2 Impact of hypocaloric diet and sibutramine on serum androstendione levels.
aP<0.05 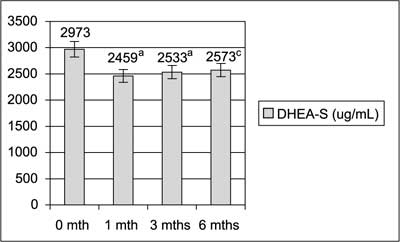 Fig. 3. Serum DHEA-S levels during treatment.
aP<0.05 cP<0.001 No patients menstruated regularly before the start of the study. Menstrual bleedings reoccurred after 1st month of treatment in fourteen women, in sixteen before 3th month and in eighteen before 6th month. In most cases (except four) patients menstruated regularly. In two subjects periods occurred after 1 month and than stopped throughout next months.
Reduction of hirsutism grade was observed only in one women after 1st month and in two women after 3 months. In only one patient acne reduction was observed throughout the study.
Sibutramine was well tolerated from all the patients.
Discussion
The present study is to our knowledge one of the only a few investigations concerning the effect of hypocaloric diet and sibutramine in women with PCOS.
Polycystic ovary syndrome is the most common endocrine disorder in premenopausal women and an important cause of oligo- and anovulatory infertility (23). Etiopathology of disease is still not fully understood and probably depends on impact of genetic, intrauterine and environmental factors with diet and lifestyle patterns among them. It is known that approximately 50% of the women with PCOS are overweight or obese (6). Some authors consider that overweight women with PCOS have central type of obesity, however estimations of the body composition have given in fact contrasting results (24-26). Central fat is metabolically active and there is a strong relationship between central obesity and insulin resistance that leads to type 2 diabetes, dyslipidemia and hypertension. In women with PCOS fat excess is also associated with abnormalities of sex steroid metabolism and is related to menstrual disorders and anovulatory infertility (27). For these reasons it seems to be of crucial importance to reduce the body weight, especially abdominal fat as a most important treatment of choice. For example the UK guidelines for the management of obese women with PCOS recommended weight loss, preferably to a BMI of less than 30 kg/m2 before starting drugs for ovarian stimulation (28). Previous studies demonstrated, that weight loss induced by lifestyle modifications (hypocaloric diet and physical activity) improves glucose and lipids metabolism, corrects endocrine abnormalities and has beneficial effects on reproduction. (29, 30). There is also a quite huge literature on pharmacological treatment of obesity in PCOS. The most extensively studied drug is metformin – insulin-sensitizing agent that remarkably reduced circulating androgens levels and body weigh (31-33). It was also proved that metformin may induce regular menstrual and ovulatory cycles (32).
In our study we intended to estimate the effects of the significant loss of body weight, especially abdominal fat on insulin resistance, androgen levels and menstruation cycles in overweight and obese women with polycystic ovary syndrome. As adherence to the lifestyle modification: dietary regiment and physical activity in long term is difficult in majority of the obese patients we added sibutramine, an agent with the excellent proved efficacy in obesity (34-36). As sibutramine in contraindicated in pregnancy all the patients before treatment were warned about this and instructed to use adequate birth control method throughout the study. No patient became pregnant during the six-months observation and next three months of the follow up.
The hypocaloric diet and sibutramine caused very significant reduction of body weight, of 12.5% (mean) during the 6 months of the study. Body weight as well as BMI and waist circumference decreased significantly already in the first month of the treatment. All these fatness parameters were decreasing further throughout the next months. Fat mass of the trunk measured twice by DEXA before and in the end of the treatment decreased of about 2.1 kg. Although total fat mass decreased of 9.2% while the fat mass of the trunk decreased of 8.2% it can't be speculated whether our treatment preferably altered abdominal fat because fat mass of the trunk include both abdominal and subcutaneous fat of all the measured area. In general, our results may indicate a very well compliance to the treatment and are fully in agreement with data from other authors (20-22).
Sibutramine added to the diet regimen caused an significant decrease in serum fasting glucose and insulin levels after 6 months of the treatment. At the same time HOMA index of insulin resistance decreased markedly although not significantly. The reasons of this not quite satisfactory result may be too short time of the observation and treatment of patients with relatively high body weight and BMI at the start of the study.
Dyslipidemia, particularly high serum total cholesterol, LDL-cholesterol and trigliycerydes is the common metabolic abnormality in PCOS (37, 38). In our study diet abidance and sibutramine resulted in significant decrease in all of these parameters already in the first months of the treatment. Moreover HDL-cholesterol increased significantly after 6 months. We didn't investigated mechanisms of this effect, although it was more likely to be a consequence of significant weight loss than a direct result of improvement in glucose metabolism, as HOMA index changed not significantly. Beneficial effects of the therapy on blood lipids, reducing the cardiovascular risk seems to be very important, despite the fact that current valid epidemiological data demonstrating an increase in clinical cardiovascular events in patients with PCOS are not available to date.
Lifestyle modification and sibutramine resulted in significant decrease in serum androgens levels. Testosterone, androstendione and DHEA-S levels decreased significantly after the first month of the treatment and were decreasing further during next month. As the hyperandrogenemia is one of the crucial hormonal disturbances in PCOS that leads to undesirable clinical effects and markedly contribute to unovulatory infertility the observed results were very important. Our results are in general in agreement with data from Sabuncu et al (20), who had observed decrease in total testosterone, free testosterone and DHEA and with data from Florakis et al (21) who had found decrease in free androgen index, in both authors after 6 months of the treatment with diet plus sibutramine.
A very impressive changes in menstrual pattern occurred throughout the study in our subjects. At the start of the treatment no women menstruated regularly. Menstrual bleedings returned after one month in fourteen women, in sixteen before 3th month and in eighteen of them before the end of the observation period. Most of the patients reported that bleedings appeared regularly. When speculating about potential mechanisms it should be remembered that weight loss has a significant effect on menstruation and ovulation (39). As it is also well known that androgen excess is one of the principal causes of menstrual and ovulatory disturbances that, even is contraindicted in pregnancy, sibutramine may be considered as the hypothetically option therapy in this group of patients. No women became pregnant during the study and in the three months of the follow up.
In general, no beneficial effects on clinical symptoms of hyperandrogenaemia (acne, hirsutism) were observed. It may be result of too short time of the treatment, too low decrease of the androgen levels and complicated etiology of the symptom (hirsutism), dependent not only on serum androgen excess.
There are several limitations in the study. First, the study was not double blind and controlled with placebo. However taking into consideration closely controlled conditions of the treatment and well known characteristics of sibutramine as the agent with the proved efficacy in obesity the close link between treatment and observed results seems to be highly probable. Second, it includes small number of patients. However relatively high correlations coefficients and low P values may confirm statistic significance of our result. Third, although we advised patients to consume a fixed calorie diet and maintain a constant level of physical activity during the study the degree of lifestyle intervention in each particular patient could be different.
Our study was finished just before the European Medicines Agency (EMEA) announced the results of its safety review of drugs containing sibutramine, citing the SCOUT data, and concluded that „the risks of these medicines are greater than their benefits” and stated that physicians should no longer prescribe sibutramine-containing agents, pharmacists should no longer dispense them, and patients taking them. With respect to the statement of EMEA we invited to the clinic all the subjects about 3 month after the end of the study for the additional visit with the cardiology examination, which did not reveal any important abnormalities.
In summary, we demonstrated that six-month treatment with diet and sibutramine led to the significant decrease in body weight and body mass index, waist circumference and fat mass, including fat mass of the trunk. Serum fasting glucose, insulin, cholesterol and triglicerydes levels decreased while HDL-cholesterol level increased significantly. Significant decrease in serum testosterone, androstendione and DHEA-S levels was observed. In almost all of the patients menstrual bleedings returned.
Conclusions
Hypocaloric diet and pharmacological treatment with sibutramine caused a significant weight loss, had beneficial effects on some metabolic and cardiovasclular risk factors, reduced androgens and restored menstruations in overweight and obese women with polycystic ovary syndrome.
**Acknowledgments: This study was supported by a grant of the Medical Center of Postgraduate Education, Warsaw, Poland; No: 501-2-1-07-48/09. Piśmiennictwo
1. Carmina E, Lobo RA: Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab 1999; 84: 1897-9.
2. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004 Revised 2003 consensus on diagnostic criteria and long-term health risk related to polycystic ovary syndrome Fertil Steril 2004; 81: 19-25.
3. Dunaif A: Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997; 18: 774-800.
4. DeUgarte CM, Bartolucci AA, Azziz R: Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 2005; 83: 1454-60.
5. Hoffman LK, Ehrmann DA: Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 2008; 4: 215-22.
6. Gambineri A, Pelusi C, Vicennati V et al.: Obesity and the polycystic ovary syndrome. Int J Obes Relat Metab Disord 2002; 26: 883-96.
7. Kirchengast S, Huber J: Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum Reprod 2001; 16: 1255-60.
8. Guzick DS: Cardiovascular risk in PCOS. J Clin Endocrinol Metab 2004; 89: 3694-5.
9. Palomba S, Orio F, Falbo A et al.: Plasminogen activator inhibitor 1 and miscarriage after metformin treatment and laparoscoping ovarian drilling in patients with polycystic ovary syndrome. Fertil Steril 2005; 84: 761-5.
10. Orio F, Giallauria F, Palomba S et al.: Cardiopulmonary impairment in young women with polycystic ovary syndrome. J Clin Endocrinol Metab 2006; 91: 2967-71.
11. Wild S, Pierpoint T, McKeigue P et al.: Cardiovascular disease in women with polycystic ovary syndrome at long-term follow up: a retrospective cohort study. Clin Endocrinol (Oxf) 2000; 52: 595-600.
12. Dagre A, Lekakis J, Mihas C et al.: Association of dehydroepiandrosterone-sulfate with endothelial function in young women with polycystic ovary syndrome. Eur J Endocrinol 2006; 154: 883-90.
13. Palomba S, Orio F, Falbo A et al.: Prospective parallel randomized, double-blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment gor ovulation induction in nonobese unovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab 2005; 90: 4068-74.
14. Lord JM, Flight IH, Norman RJ: Metformin in polycystic ovary syndrome: systematic review and metaanalysis. BMJ 2003; 327: 951-3.
15. Farquhar C, Vandekerckhove P, Lilford R: Laparoscopic "drilling” by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochane Database Syst Rev 2001; CD001122.
16. Bayram N, van Wely M, Kaaijk EM et al.: Using an electrocautery strategy or recombinant follicle stimulating hormone to induce ovulation in polycystic ovary syndrome: randomized controlled trial. BMJ 2004; 328: 192.
17. Legro RS, Barnhart HX, Schlaff WD et al.: Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356: 551-66.
18. Costello MF, Eden JA: A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil Steril 2003; 79: 1-13.
19. Kashyap S, Wells GA, Rosenwaks Z: Insulin-sensitizing agents as primary therapy for patients with polycystic ovary syndrome. Hum Reprod 2004; 19: 2474-83.
20. Sabuncu T, Harma M, Harma M et al.: Sibutrmine has a positive effect on clinical and metabolic parameters in obese patients with polycystic ovary syndrome. Fertil Steril 2003; 80: 1199-204.
21. Florakis D, Diamanti-Kandarakis E, Katsikis I et al.: Effect of hypocaloric diet plus sibutramine treatment on hormonal and metabolic features in overweight and obese women with polycystic ovary syndrome: a randomized, 24-week study. Int J Obes 2008; 32: 69-9.
22. Lindholm A, Bixo M, Björn I et al.: Effect of sibutramine on weight reduction in women with polycystic ovary syndrome: a randomized, double-blind, placebo controlled trial. Fertil Steril 2008; 89: 1221-8.
23. Hull MG: Epidemiology of infertility and polycystic ovarian disease: endocrinological and demographic studies. Gynecol Endocrinol 1987; 1: 235-45.
24. Yildrim B, Sabir N, Kaleli B: Relation of intra-abdominal fat distribution to metabolic disorders in nonobese patients with polycystic ovary syndrome. Fertil Steril 2003; 79: 1358-64.
25. Faloia E, Canibus P, Gatti C et al.: Body composition, fat distribution and metabolic characteristics in lean and obese women with polycystic ovary syndrome. J Endocrinol Invest 2004; 27: 424-9.
26. Puder JJ, Varga S, Kraenzlin M et al.: Central fat excess in polycystic ovary syndrome: relation to low grade inflammation and insulin resistance. J Clin Endocrinol Metab 2005; 90: 6014-21.
27. Kiddy DS, Sharp PS, White DM et al.: Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin Endocrinol (Oxf) 1990; 32: 213-20.
28. National Institute for Clinical Excellence. Fertility assessment and treatment for people with fertility problems. A clinical guideline. London 2004; RCOG Press.
29. Norman RJ, Noakes M, Wu R et al.: Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update 2004; 10: 267-80
30. Pasquali R, Gambineri A, Pagotto U: The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG 2006; 13: 1148-59.
31. Kjotrod SB, Sunde A, Düring VV et al.: Possible metformin effect on adrenal androgens during pretreatment and IVF cycle in women with polycystic ovary syndrome. Fertil Steril 2009; 91: 500-8.
32. Velazques EM, Mendosa S, Hamer T et al.: Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenaemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994; 43: 647-54.
33. Palomba S, Falbo A, Zullo F et al.: Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocrine Rev 2009; 30: 1-50.
34. James WPT, Astrup A, Finer N et al.: Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Lancet 2000; 356; 2119-25.
35. Waden TA, Berkowitz RI, Leslie G et al.: Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 2005; 353: 2111-20.
36. Tziomalos K, Krassas GE, Tzotzas T: The use of sibutramine in the management of obesity and related disorders: an update. Vasc Health Risk Manag 2009; 441-52.
37. Hofman LK, Ehrmann DA: Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 2008; 4: 215-22.
38. Rizzo M, Berneis K, Carmina E et al.: How should we manage atherogenic dyslipidemia in women with polycystic ovary syndrome? Am J Obstet Gynecol 2008; 198: 28.e1-e5.
39. Clark AM, Ledger W, Galletly C et al.: Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum Reprod 1995; 10: 2705-12.
otrzymano/received: 2010-06-18 zaakceptowano/accepted: 2010-07-20 Adres/address: *Jarosław Kozakowski Department of Endocrinology, Medical Center of Postgraduate Education, Bielański Hospital ul. Cegłowska 80, 01-809 Warsaw tel.: +48 (22) 834-31-31, fax: +48 (22) 834-31-31 e-mail: kyaroslaw@tlen.pl |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








