|
© Borgis - Postępy Nauk Medycznych 3, s. 195-198
*Olga Kozińska-Przybył, Andrzej Mróz, Magdalena Durlik
Hemolytic uremic syndrome (HUS) After Kidney Transplantation in Two Patients Treated With Sirolimus – case report
Zespół hemolityczno-mocznicowy u dwóch biorców przeszczepu nerkowego leczonych sirolimusem – opis przypadków
Klinika Medycyny Transplantacyjnej i Nefrologii Instytutu Transplantologii Warszawskiego Uniwersytetu Medycznego
Kierownik Kliniki: prof. dr hab. med. Magdalena Durlik Streszczenie
Sirolimus, m-TOR inhibitor, poprzez wplyw na obnizenie VEGF w nerkach przeszczepionych, dzialanie proagragacyjne, moze wywolac rozwój mikroangiopatii zakrzepowej. Manifestacja kliniczna jest wówczas zespól hemolityczno-mocznicowy charakteryzujacy sie niedokrwistoscia hemolityczna, maloplytkowoscia oraz niewydolnoscia nerki przeszczepionej. Ponizej prezentujemy dwa przypadki pacjentów po zabiegach przeszczepienia nerek, którzy rozwineli zespól hemolityczno-mocznicowy podczas terapii sirolimusem. Zaden nie mial w wywiadzie zespolu hemolityczno-mocznicowego jako przyczyny niewydolnosci nerek wlasnych. Rozpoznanie mikroangiopatii zakrzepowej potwierdzono badaniem histopatologicznym wycinka nerki przeszczepionej pobranej podczas biopsji cienkoiglowej. Pomimo istnienia wielu czynników mogacych wplynac na rozwiniecie mikroangiopatii zakrzepowej jak: drugie przeszczepienie, leczenie takrolimusem, wysokie PRA (przypadek 1 i 2), DGF, poprzedzajace ostre odrzucanie humoralne (przypadek 2), dlugi czas niedokrwienia oraz starszy wiek dawcy ( przypadek 1), dopiero odstawienie rapamycyny poskutkowalo poprawa czynnosci przeszczepu. Potwierdza to teze o szkodliwym wplywie m-TOR inhibitorów na sródblonek naczyniowy w okresie ostrej mikroangiopatii zakrzepowej. Słowa kluczowe: mikroangiopatia zakrzepowa, zespól hemolityczno-mocznicowy, biopsja, sirolimus, odrzucanie naczyniowe
Summary
Sirolimus by its ability to lower VEGF in transplanted kidney and promotion of platelet aggregation can predispose to thrombotic microangiopathy (TMA), which can be severe. We identify two cases of TMA in renal transplant recipients, who were being treated with sirolimus. Clinically patients demonstrated hemolytic uremic syndrome. Neither patient had evidence of TMA as primary disease in native kidney. Diagnosis was based on histopathologic examination of renal graft specimen, C4d stainig was also performed. Both had additional risk factors for TMA development like: previous renal transplant, high PRA, treatment with tacrolimus (case 1 and 2), DGF, preceding humoral rejection episode (case 2), older donor age and long ischemic time. Only with the discontinuation of sirolimus showed renal graft improvement. This agrees with the theory that sirolimus has an damaging effect on endothelial cells, moreover in cases with acute TMA. Key words: thrombotic microangiopathy, hemolytic-uremic syndrome, sirolimus, biopsy, humoral rejection
Introduction
HUS consisting of hemolytic anemia, thrombocytopenia, and acute renal failure, is clinical manifestation of thrombotic microangiopathy (TMA) diagnosed by histopathologic examination of a biopsied kidney specimen. It occurs in 5-15% of patients who receive cyclosporine and in approximately 1-5% of those who are given tacrolimus (6, 7). TMA is being reported with increasing frequency (8) also in patients receiving sirolimus-based immunosuppressant drugs regimen (9). Recent experimental studies showed that sirolimus itself can cause thrombotic microangiopathy and delay healing process after TMA. We present cases of two patients who developed HUS after treatment of combined FK506/ATG/Sirolimus regimen and fully recovered after discontinuation of sirolimus.
Case #1
26-years old patient with end stage renal failure caused by chronic pyelonephritis underwent second cadaver renal allograft from 50 year old donor. Due to high immunisation (max. PRA was 74%, last 36%) immunosupression was started with ATG – fresenius (1-st dose 9 mg/kg), methylprednisolone (250 mg for day 0), tacrolimus (initially 0.2 mg/kg per day) and sirolimus ( first dose 6 mg). ATG was reduced to the dose of 3 mg/kg (3 doses) under the control of number of CD 3 plus cells. The graft functioned immediately and serum creatinine dropped from 4 mg/dl to 3.3 mg/dl on post-operative day 6. From that time, no further improvement in graft function was observed. On day 13 post-transplant, graft function started to deteriorate (serum creatinine 3.8 mg/dl) with normal graft perfusion on colored doppler study. Anemia and thrombocytopenia were primary considered to be consistent with ATG myelotoxicity (fall in hemoglobin 9.3-6.2 g/l; fall in platelet count 254-76 x 10 9/l). Whole blood 12-h through tacrolimus, and 24-h through levels of sirolimus were within therapeutic ranges (FK: min. 7.2 ng/dl, max. 10.2 ng/dl, Rapa 4.5). There were no biochemical signs of hemolysis, however there were high LDH levels (1554 U/L, normal range 80-248 U/L). In the first core biopsy vascular changes can be seen. Nearly total occlusion of majority of arteries due to non-inflammatory mucoid-like intimal proliferation was observed (fig. 1). C4d deposits were found; additionally in some vessels homogenic acidophilic deposits interpreted as fibrin were present. These changes of thrombotic angiopathy were not accompanied by acute rejection nor were remarkable chronic interstitial features found. The clinical picture of intravascular haemolysis, thrombocytopenia, and worsening renal function with microangiopathy in histopathology examination was primary assumed to be tacrolimus – inducted haemolytic-uremic syndrome (HUS). Tacrolimus was discontinued and mycophenolate mophetil was started in a dose of 1.0 g per day then raised to 2.0 g per day. Neither plasmapheresis nor fresh frozen plasma was employed, instead methylprednisolone was given as a prophylaxis of acute rejection in highly immunized patient (3 x 500 mg, then 10 x 125 mg). There was no renal graft function improvement; however whole blood sirolimus level was maintained on 3.0 ng/ml. The second biopsy performed on 25 day displayed similar arterial changes of mucoid-like intimal proliferation with nearly complete occlusion of vascular lumen, so the diagnosis of thrombotic angiopathy was sustained (fig. 2), C4d deposits were not found. Vascular disturbances resulted in significant ischaemia that led to cortical necrosis, therefore sirolimus was discontinued and fast normalization of renal function was observed (fall in creatinine to 1.8 mg/dl).
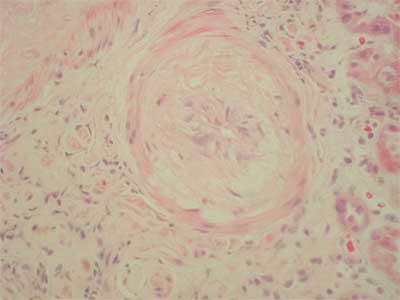 Fig. 1. Arterial intimal mucoid-like proliferation.
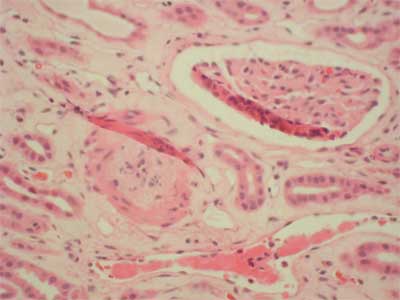 Fig. 2. Arterial intimal proliferation.
Case #2
34-years old patient with end-stage renal failure secondary to chronic glomerulonephritis (membrano-proliferative), high PRA (max. 86%, last 53%), underwent two matched HLA antigens, second cadaveric kidney transplantation from 29 year old donor. First, renal allograft treated with prednisone and azathioprine functioned for 8 months, however it failed because of unknown process. Intra-operatively, the patient received 275 mg i.v. methylprednisolone, ATG 9 mg/kg, tacrolimus 0.1 mg/kg per os, sirolimus 6 mg. Post-operatively, ATG dose was reduced to 3 mg/kg (3 doses). The graft did not function immediately and the patient required several hemodialysis's. Acute humoral rejection (IIA) was observed in renal graft biopsy and methlprednisolone pulse therapy (3 x 250 mg) was given with slow, but significant improvement in graft function (serum creatinine dropped from 8 mg/dl to 2.4 mg/dl at day 45). On the 51st day post-transplant platelet count began to fall (203 to 75 x 109/l) along with hemoglobin (9.7-7.9 g/l), but a rise in serum creatinine (2.4-4.7 mg/dl) and LDH (1440-1932 u/L.). The core biopsy displayed florid microthrombotic features of fibrin thrombi occluding glomerular capillaries and arterioles C4d was negative (fig. 3). Apart from the toxic etiology the possible component of acute vascular rejection was also pointed due to the presence of inflammatory cells touching or infiltrating under the activated endothelium (fig. 4). There was no interstitial change observed, also no evidence of CMV infection. Whole blood tacrolimus levels were within range (5.8-12.5 ng/ml at day 45 postransplant). Sirolimus blood level was 4.0 ng/ml FK 506 was discontinued immediately, MMF and fresh frozen plasma was given. Whole blood tacrolimus level measured 36 hours after its withdrawal was 10.8 ng/ml and plasmapheresis was not preformed. 3 doses of 0.5 g of metylprednisolone and 10 doses of 125 mg were administered. Serum creatinine fell to 3.2 mg/dl, however, very high LDH level (2003 U/L.) and thrombocytopenia were observed (69 x 109/L). There was no show of further improvement, therefore sirolimus was discontinued. Laboratory findings characteristic for HUS started to normalize (LDH 1465, platelet count 167 x 109), further deterioration in graft function was observed and therefore a core biopsy was performed. In contrast to previous pictures no features of active thrombotic process were present in the biopsy. The diagnosis of borderline changes (focal interstitial infiltrates and tubulitis) coexisted with moderate tubular atrophy, arterial intimal fibrosis, and interstitial fibrosis. These changes were more advanced than in the previous biopsy. Additionally, ischaemic glomerular changes were present; the patient was treated with 3 x 0.5 g methylprednisolone and the dose of MMF was increased to 3 g per day which caused the serum creatinine to fall to 1.7 mg/dl
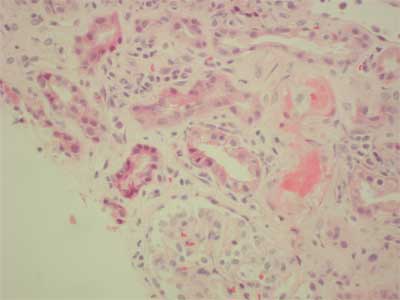 Fig. 3. Arterioral thrombi.
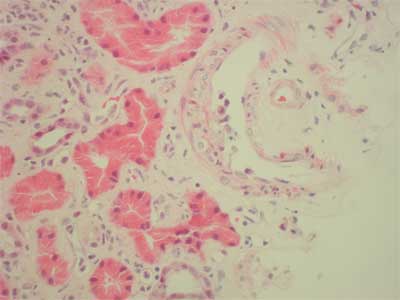 Fig. 4. Inflammatory cells approaching activated endothelium.
Discussion
Pathogenesis of developing thrombotic microangiopathy after renal transplantation is complex. The main role in etiology plays damage of endothelium, thus all factors that cause endothelial cell injury may influence formation of thrombus in microvasculature of transplanted kidney. They include not only immunosuppressive drugs like calcineurin-inhibitors (CI – cyclosporine, tacrolimus), ATG, OCT3 but, also bacterial, viral infections, and processes of humoral rejections. One of therapeutic options can be in some cases conversion to sirolimus (SRL) (1). On the other hand m-TOR inhibitors may predispose to thrombotic microangiopahty development by decreasing of vascular endothelial growth factor (VEGF) expression in human transplanted kidney (2) and platelet aggregation (13). The loss of function (VEGF) through genetic depletion, pharmacologic inhibition, or an elevated level of circulating soluble forms that binds VEGF is associated with damage to the glomerular endothelium (2). Letavernier et al. shows that WT1 gene – a transcription factor essential for maintaining podocyte integrity and protein expression in podocytes were decreased in dose dependent manner after incubation with sirolimus (3).
In both cases, not only final diagnosis of TMA, but suggested that in multiple previous reports (9, 11, 12) relationship between SRL and development of TMA were confirmed in histopathologic examinations. In case #1 changes characteristic for thrombotic microangiopathy persisted after discontinuation of CI; in case #2 it lacked these changes after discontinuation of SRL. Although the pathogenesis of HUS seems to be more complex; both patients had additional risk factors according to large analysis of United States Renal Data System (USRDS) for developing of de novo TMA like: previous renal transplant, increased peak panel-reactive antibody and treatment with CNI. Moreover, older donor age, long C IT (more than 30 hours) in the case #1 and preceding humoral rejection episode together with DGF in case #2 – are also well defined risk factors leading to HUS. In both cases characteristic for TMA histopatological changes coexisted with clinical signs of hemolysis; although in the case #1 close temporal relationship between ATG administration and developing of anemia and thrombocytopenia was first considered as ATG side effect. In case #1 no characteristic changes for coexisting rejection were observed, although C4d staining was positive it might be due to ischemic injury during transplantation (10). Methylprednisolne was administrated as a prevention of acute rejection, and fast clinical improvement was observed. Case #2 is more complex due to rejection episodes. After SRL discontinuation renal improvement was not observed, although biochemical signs of hemolysis started to normalize. Control biopsy showed only borderline changes, which were treated with metylprednisolone and intensification of immunosupression with success.
Conclusions
Sirolimus by its ability to lower VEGF in transplanted kidney and promotion of platelet aggregation (13) can predispose to HUS development, which can be severe. In such cases m-TOR inhibitor should be discontinued. Saterlet et al. shows that m-TOR inhibitors should not be used during active microangiopathy, acute humoral rejection, and other situation of renal diseases with predominant endothelial cell injury. However it appears to be safe once acute injury has subsided (5). This theory could explain successful conversion from CI to sirolimus in cases with mild syndrome. Piśmiennictwo
1. Franco A, Hernandez D, Capdevilla L et al.: De novo hemolytic uremic syndrome/thrombotic microangiopathy in renal transplant patients receiving calcineurin inhibitors; role of sirolomus. Transpl Proc 2003; 35: 1764-1766.
2. Sartelet H, Toupance O, Lorenzato M et al.: Sirolimus-inducted thrombotic microangiopaty is associated with decreased expression of vascular endothelial growth factor in kidneys. Am J Transplt 2005; 5: 2441-2447.
3. Letavernier E, Bruneval P, Vandermeersch S et al.: Sirolimus interacts with pathways essential for podocyte integrity. Nephrology, Dialysis, Transplantation. Eynsham: Feb 2009; 24: 630.
4. Trimarchi HM, Truong LD, Brennan S et al.: FK – 506 associated thrombotic microangiopathy: report of two cases and review of the literature. Transplantation 1999; 67: 539-544.
5. Keller K, Daniel Ch, Schocklmann H et al.: Everolimus inhibits glomerular endothelial proloferation and VEGF, but not long-term recovery in experimental thrombotic microangiopaty. Nephrol Dial Transplt 2006; 21: 2724-2735.
6. Zafiran A, Meleg-Smith S, ODonovan R et al.: Cyclosporine-associated thrombotic microangiopathy in renal allografts. Kidney Int 1999; 55: 2457-66.
7. Ruggeneti P: Post-transplantation hemolitic uremic syndrome. Kidney Int 2002; 62: 1093-104.
8. Reynold JC, Agodoa LY, Yuan CM et al.: Thrombotic microangiopathy after renal tranpslantation in the United States. Am J Kidney Diss 2003; 42: 1058-68.
9. Barone GW, Gurley BJ, Abul-Ezz SR: Gokden N Sirolimus-inducted thrombotic microangiopathy in a renal transplant recepipients. Am J Kidney Dis 2003; 42: 202-206.
10. de Vries B, Walter SJ, Peutz-Kootstra CJ et al.: The mannose-binding lectin-pathway is involved in complement activation in the course of renal ischemia-reperfusion injury. Am J Pathol 2004; 165: 1677-88.
11. Robson M, Cote I, Abbs I et al.: Thrombotic micro-angiopathy with sirolimus-based immunosupresion: potentation of calcyneurin-inhibitor-inducted endothelial damage? Am J Transplant 2003; 3: 324-327.
12. Saikali JA, Truong LD, Suki WN: Sirolimusmay promotethrombotic microangiopathy. Am J Transplant 2003; 3: 229-230.
13. Babinska A, Markell MS, Salifu MO et al.: Enhancement of human platelet aggregation and secretioninducted by rapamycin. Nephrol Dial Transplant 1998; 13: 3153-9.
otrzymano/received: 2010-01-21 zaakceptowano/accepted: 2010-02-19 Adres/address: *Olga Kozińska-Przybył Klinika Medycyny Transplantacyjnej i Nefrologii Instytutu Transplantologii Warszawskiego Uniwersytetu Medycznego ul. Nowogrodzka 59, 02-006 Warszawa tel.: 0 605-783-827 e-mail: okp7@wp.pl Artykuł Hemolytic uremic syndrome (HUS) After Kidney Transplantation in Two Patients Treated With Sirolimus – case report w Czytelni Medycznej Borgis. |
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








