|
© Borgis - Postępy Nauk Medycznych 1, s. 10-14
*Bożena Walewska-Zielecka
Ekspresja antygenów wirusa C zapalenia wątroby i aktywność zapalna w wątrobie u osób przewlekle zakażonych HCV
HCV antigens expression and inflammatory activity in patients with chronic HCV infection
National Institute of Public Health, National Institute of Hygiene, Department of Virology
Head of Department: Bogumiła Litwińska Streszczenie
Przewlekłe zapalenie wątroby jest istotnym czynnikiem wpływającym na globalne problemy zdrowotne. Szacuje się, że mniej niż 30% zakażonych HCV eliminuje wirusa. U pozostałych 70-80% zakażonych dochodzi do przewlekłego zapalenia wątroby, marskości i jej różnych powikłań. Celem pracy było zbadanie częstości występowania antygenów wirusa C zapalenia wątroby (HCVAgs) i porównanie występowania ekspresji HCVAgs z całkowitą aktywnością zapalną w wątrobie u osób przewlekle zakażonych tym wirusem. Materiał badany stanowiły wycinki z biopsji wątroby 137 pacjentów przewlekle zakażonych HCV. Antygeny HCV w wątrobie wykrywano w skrawkach mrożonych metodą EnVision z zastosowaniem przeciwciał ludzkich znakowanych FITC. HCVAgs wykryto w 67 spośród 137 badanych próbek (48,9%). Całkowita aktywność zapalna nie korelowała z ekspresją antygenów HCV. Stwierdzono jednak większą ekspresję antygenów HCV w obszarach miejscowo nasilonego odczynu zapalnego. Rozszerzenie diagnostyki histopatologicznej o badanie immunomorfologiczne antygenów HCV w wątrobie może przyczynić się do pogłębienia wiedzy o immunopatogenezie przewlekłych zapaleń wątroby i może być wartościowym badaniem uściślającym etiologię przewlekłego zapalenia wątroby. Słowa kluczowe: antygeny HCV, biopsją wątroby, badanie immunohistochemiczne
Summary
Chronic type C hepatitis remains a substantial burden to global health problems. It is estimated, that only up to 30% of infected persons effectively eliminate the virus. In the remaining 70-80% chronic infection develops, with threat of all consequences. The aim of the study was to establish the expression rate of HCV antigens (HCVAgs) in liver tissue and to compare it with the degree of inflammatory reaction in the liver of patients chronically infected with HCV. Liver biopsy specimens from 137 patients with chronic HCV infection were studied. The expression of HCV antigens was detected in frozen liver tissue sections by EnVision, DAKO with the use of FITC-labeled human antibodies to HCV antigens. HCVAgs were detected in 67 out of 137 of the specimens examined (48.9%). Total inflammatory activity did not correlate with HCV antigens expression, however local inflammatory reaction was found as a response to abundant HCVAgs expression. The extension of histopathological examination by immunohistochemical analysis of HCVAgs in liver tissue may contribute to broadening of the knowledge of the immunopathogenesis of chronic hepatitis and may be regarded as valuable tool for searching for chronic hepatitis etiology. Key words: HCV antigens, liver biopsy, immunohistochemistry
Chronic type C hepatitis remains a substantial burden to global health problems. According to recent data there are 130 million people infected with HCV, and global prevalence is 2.2% (1). Based on WHO data the prevalence of HCV in Poland is reported as 1% (2). However, Polish experts estimate HCV prevalence in Poland at 1.9%, i.e. about 730 000 persons infected with HCV (3). It is known, that only up to 30% of infected persons effectively eliminate this virus, in the remaining 70-80% patients chronic infection develops, with threat of all clinical consequences (chronic hepatitis, liver cirrhosis and hepatocellular carcinoma) (3, 4, 5, 6).
Histopathological examination of needle biopsy specimen has been regarded as a "gold standard” in the diagnostic procedure of chronic hepatitis of different etiology. The type of liver pathology, the grade of inflammatory activity and the stage of liver fibrosis is evaluated by microscopic analysis of liver tissue (7, 8, 9). The search for tissue expression of HCV antigens seems to be a new and promising diagnostic method, but actually remains restricted mostly to research in basic sciences. Up to now HCV antigens (HCVAgs) were localised exclusively in the cytoplasm of hepatocytes, both in acute and chronic hepatitis in chimpanzees and in humans (10-19).
Inflammatory infiltrate present in a needle biopsy specimen is regarded as evidence of immunological processes in situ, in reaction to viral protein expression. Host attempts to eliminate viruses lead to damage and subsequent necrosis of hepatocytes (20-22). Damage of infected hepatocytes is a result of immune reactions aimed at eliminating infection. Therefore, necrosis of hepatocytes and mononuclear cells reaction is a key feature of this process. The intensity of the immune reaction in liver tissue depends both on the immunological status of the host and immunogenicity of HCV proteins expressed in the liver (5, 6, 23).
In this study the expression of HCVAgs in the liver of patients with chronic HCV infection was evaluated. The results were compared with inflammatory activity in the liver tissue.
Material and methods
The material studied consisted of single liver biopsy specimens and serum samples from 137 patients with chronic liver disease of HCV etiology (mean age 41; range 9-73 yrs) referred to our Department for histopathological and molecular evaluation. HCV etiology of liver disease was confirmed by clinical and serological criteria (anti-HCV and HCV RNA). No other underlying liver disease was present in these patients.
Part of each biopsy specimen (at least 12 mm length) was fixed in formalin and routinely processed to paraffin; the remaining part was divided into two fragments both rapidly frozen. One fragment, measuring approximately 5-6 mm in length was kept at -40°C until used for the detection of antigens, and the other one measuring approximately 2 mm in length was kept at -65°C for RNA extraction subsequent to homogenization.
Histopathologic diagnosis of chronic hepatitis, that included grading and staging of the process (7, 24), was established by examination of serial paraffin sections stained with hematoxylin-eosin, chromotrope 2R-aniline blue and impregnated with silver according to Gomori (25, 26). Total inflammatory activity was ascertained by histopathological activity index (tab. 1). To avoid the overestimation of fibrosis Gomori stain was applied which differentiate reticulin and collgen fibers, therefore shows the difference between reticulin fibres collapse and collagen deposits. The numerical score was presented as HAI (histopathological activity index) (7, 24).
Table 1. Histopathological evaluation and scoring system applied in this study. Modified Knodell score (Histopathological Activity Index; HAI) stratification and fibrosis evaluation (7, 24).
A. Inflammatory activity
B. Fibrosis score
HCVAgs were detected with the use of human FITC-labeled polyclonal anti-HCV antibodies (12), followed by monoclonal anti-fluorescein antibodies, and finally, by anti-mouse globulin antibodies labeled with peroxidase (EnVision system, DAKO). The specificity of the methods applied in this study was ascertained by negative staining results when primary antibody was omitted or replaced by animal or human sera that did not contain antibodies against antigens of HCV. Direct inflammatory response to viral antigens expression was evaluated during immunohistochemical examination of liver tissue.
Results
HCVAgs were detected in 67/137 cases (48.9%). These antigens were found exclusively in the cytoplasm of hepatocytes as amorphous deposits or small granules (fig. 1). Local inflammatory response, both intralobular (spotty necrosis) and periportal developed in close vicinity of hepatocytes with HCVAgs expression. Particularly numerous cells expressing HCVAgs were found in hepatocellular carcinoma occurring in liver cirrhosis caused by HCV (fig. 2).
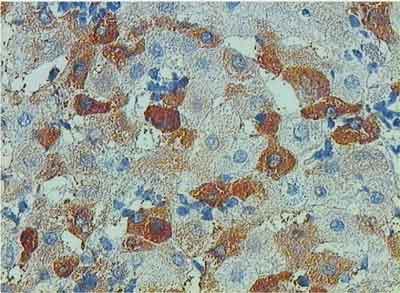 Fig. 1. Expression of HCV Ags in numerous hepatocytes. Inflammatory mononuclear cells in direct vicinity of hepatocytes. EnVision, x200.
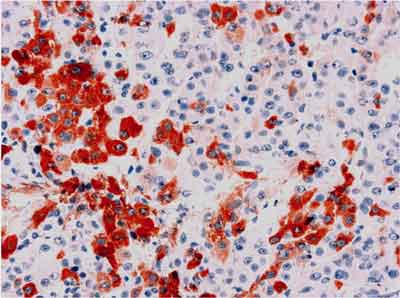 Fig. 2. Expression of HCVAgs in hepatocellular carcinoma. EnVision, x125.
Inflammatory activity ascertained by modified Knodell score revealed chronic hepatitis in all biopsies examined (even in patients with normal aminotransferases activity and in patient with hepatocellular carcinoma in other parts of the liver). The stratification of scores corresponding to overall activity is presented in table 2. Fibrosis usually paralleled degree of inflammation (fig. 3).
Table 2. Activity of chronic type C hepatitis in 137 liver biopsy specimens.
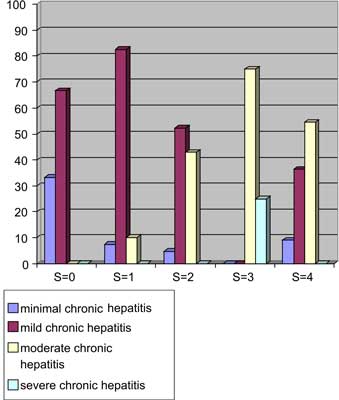 Fig. 3. Fibrosis ( staging) and degree of inflammatory activity ( grading) in chronic HCV infection. Columns display the percentage of cases diagnosed. For inflammatory activity score and fibrosis score, see table 1.
The comparison of HCVAgs expression with overall inflammatory activity in the liver is shown on figure 4 and in table 3. It was observed that HAI index did not correlate with the presence of HCVAgs.
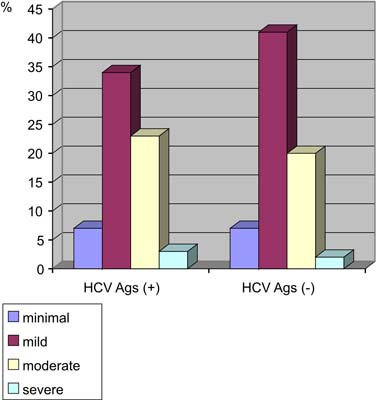 Fig. 4. Frequency of the different grades of inflammatory activity in liver tissue with – and without HCVAgs.
Table 3. Frequency of HCVAgs in liver tissue specimens with different inflammatory activity (HAI).
Discussion
In English written literature the frequency of HCVAgs expression in liver biopsy samples from patients with chronic type C hepatitis ranges from 23% to 93% (10-19), and it reaches 100% in both, acute type C hepatitis in humans and in experimentally infected chimpanzees (11-13). In the present study HCV antigens were detected in almost 50% of chronic type C viral infection positive for HCV RNA in the liver.
The differences in HCV antigens detection rate may result from the type of anti-HCV antibody applied, the method of tissue fixation, the procedure of staining, and the degree of viral protein expression (11, 12). The detection of HCV viral antigens is difficult due to very weak expression of those proteins in hepatocytes. In the present study, to detect HCV antigens we applied naturally acquired human antibody and one of the most sensitive multi-step detection systems (EnVision). The relatively low detection rate could possibly be attributed to the apparently low levels of HCV genome in the liver of patients studied and HCV genome products, as the majority of cases were diagnosed as minimal or mild chronic hepatitis. Obtained figure of 48% positivity of HCVAgs is probably underestimated, because of the fact that detection of this antigen was dependent on the amount of sections investigated (the more slides were stained, the more possibilities to find HCVAgs – in few questionable cases HCVAgs were found in single hepatocytes after serial sectioning of whole material).
The presence of HCV antigens in a half of the examined specimens may suggest that testing of liver biopsy specimens for HCV antigens may, in a significant proportion of cases, extend the histopathological diagnosis as to include identification of the etiologic factor.
In present study no correlation of HCVAgs expression and overall inflammatory activity was found. However detailed immunohistological evaluation showed local inflammatory response (either intralobular or periportal) in vicinity of hepatocytes with HCV antigen expression.
The HCV antigen expression was not evenly distributed. The length of liver specimen increased the number of cells expressing viral antigens. This unevenness of viral proteins expression might be the equivalent of focal viral nucleic acid synthesis. Therefore studies of inflammatory cells derived from peripheral blood or extracted from liver tissue reflect "averaged” information on type of immune reaction (as reflected by phenotype of inflammatory cells or lymphokine production by T lymphocytes in situ (28, 29). It cannot be excluded that the type of immunological reaction (Th1 vs Th2) is localized and focal. It reflects the variety of immunological processes taking place in the liver – directed either on elimination of virus or on tolerance of proteins synthesized by hepatotropic virus locally. Napoli et al. showed no correlation of viral load by PCR in the liver and inflammation intensity (29).
The fact of uneven distribution of inflammation and fibrosis is the reason of criticism of semi-quantitative scoring systems. It demands adequate size of liver biopsy sample for proper histological evaluation (20 mm in length in adult patients) (30).
Recent development of non-invasive tests for staging and grading of hepatitis are useful, however liver biopsy still is considered as important diagnostic tool, especially in determining prognosis and supporting therapeutic decisions (30).
Extension of classical histology by HCV antigens detection in liver tissue may be a valuable tool for confirmation of viral etiology of liver disease and also may contribute to knowledge broadening of the immunopathogenesis of chronic hepatitis. Piśmiennictwo
1. Alter MJ: Epidemiology of hepatitis C infection. World J Gastroenterol 2007; 13: 2436-2441.
2. Alter HJ, Seef LB: Recovery, persistence, and sequelae in hepatitis C infection: a perspective on long-term outcome. Sem Liver Disease 2000; 20: 17-35.
3. Strona internetowa Polskiej Grupy Ekspertów www.pgehcv.pl
4. Michielsen PP, Francque SM, Van Dongen JL: Viral hepatitis and hepatocellular carcinoma. Review. World J Surg Oncol 2005; 3: 27-45.
5. Cerny A, Chisari FV: Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 1999; 30: 595-601.
6. Chisari FV, Ferrari C: Viral Hepatitis. [In:] Viral Pathogenesis. Edit.: Nathanson N, Ahmed R, Gonzalez-Scarano F, Griffin DE, Holmes KV, Murphy FA, Robinson HL, Lippincot-Raven Publishers, Philadelphia,1997; 745-778.
7. Desmet VJ, Gerber M, Hoofnagle JH et al.: Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994; 19: 1513-1520.
8. Ishak K, Baptista A, Bianchi L et al.: Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22: 696-699.
9. Scheuer PJ: Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 1991; 13: 372-374.
10. Verslype C, Nevens F, Sinelli N et al.: Hepatic immunohistochemical staining with a monoclonal antibody against HCV-E2 to evaluate antiviral therapy and reinfection of liver grafts in hepatitis C viral infection. J Hepatol 2003; 38: 208-214.
11. Scheuer PJ, Krawczynski K, Dhillon AP: Histopathology and detection of hepatitis C virus in the liver. Springer Semin Immunopathol 1997; 19: 27-45.
12. Krawczynski K, Beach MJ, Bradley DW et al.: Hepatitis C virus antigen in hepatocytes: immunomorphologic detection and identification. Gastroenterology 1992; 103: 622-629.
13. Sansonno D, Iacobelli AR, Cornacchiulo V et al.: Immunohistochemical detection of hepatitis C virus-related proteins in liver tissue. Clin Exp Rheumatol 1995; 13 (Suppl. 1): S29-S32.
14. Ballardini G, Groff P, Giostra F et al.: Hepatocellular codistribution of c100, c33, c22, and NS5 hepatitis C virus antigens detected by using immunopurified polyclonal spontaneous human antibodies. Hepatology 1995; 21: 730-734.
15. Dries V, Von Both I, Műller M et al.: Detection of hepatitis C virus in paraffin-embedded liver biopsies of patients negative for viral RNA in serum. Hepatology 1999; 29: 223-229.
16. Hiramatsu N, Hayashi N, Haruna Y et al.: Immunohistochemical detection of hepatitis C virus-infected hepatocytes in chronic liver disease with monoclonal antibodies to core, envelope and NS3 regions of the hepatitis C virus genome. Hepatology 1992; 16: 306-311.
17. Nouri-Aria KT, Sallie R, Mizokami M et al.: Intrahepatic expression of hepatitis C virus antigens in chronic liver disease. J Pathol 1995; 175: 77-83.
18. Yamada G, Nishimoto H, Endou H et al.: Localization of hepatitis C viral RNA and capsid protein in human liver. Dig Dis Sci 1993; 38: 882-887.
19. Yap SH, Willems M, Van den Oord J et al.: Detection of hepatitis C virus antigen by immuno-histochemical staining: a histological marker of hepatitis C virus infection. J Hepatol 1994; 20: 275-281.
20. Lechner F, Wong DK, Dunbar PR et al.: Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med 2000; 191: 1499-1512.
21. Rehermann B, Nascimbeni M: Immunology of hepatitis B virus and hepatitis C virus infection. Nature Reviews 2005; 5: 215-229 [PMID: 15738952].
22. Thimme R, Oldach D, Chang KM et al.: Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med 2001; 194: 1395-1406.
23. Wedemeyer H, He XS, Nascimbeni M et al.: Impaired effector function of hepatitis C virus specific CD8- T cells in chronic hepatitis C virus infection. J Immunol 2002; 169: 3447-3458.
24. Knodell RG, Ishak KG, Black WC et al.: Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981; 1: 431-435.
25. Bianchi L, Spichtin HP, Gudat F: Chronic hepatitis. In: MacSween RNM, Anthony PP, Scheuer PJ. Pathology of the Liver. Edinburgh: Churchill Livingstone 1987; 310-343.
26. Scheuer PJ, Lefkowitch JH: Liver Biopsy Interpretation. Laboratory Techniques. London: W.B. Saunders 1994; 10-15.
27. Radkowski M, Gallegos-Orozco JF, Jablonska J et al.: Persistence of hepatitis C virus in patients successfully treated for chronic hepatitis C. Hepatology 2005; 41(1): 106-14.
28. Agrati C, D'Offizi G, Narciso P et al.: Vδ1 T lymphocytes expressing a Th1 phenotype are the major γδ T cell subset infiltrationg the liver of HCV-infected persons. Molecular Med 2001; 7: 11-19.
29. Napoli J, Bishop GA, McGuinness PH et al.: Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology 1996; 24: 759-765.
30. Rockey DC, Caldwell SH, Goodman ZD et al.: Liver biopsy. AASLD Position Paper, l Hepatology 2009; 49: 1017-1044.
otrzymano/received: 2009-10-30 zaakceptowano/accepted: 2009-12-04 Adres/address: *Bożena Walewska-Zielecka National Institute of Public Health, National Institute of Hygiene, Department of Virology 24 Chocimska Str., 00-791 Warsaw tel.: +48 (22) 542-13-37 e-mail: bwz10@wp.pl Artykuł Ekspresja antygenów wirusa C zapalenia wątroby i aktywność zapalna w wątrobie u osób przewlekle zakażonych HCV w Czytelni Medycznej Borgis. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








