|
© Borgis - Postępy Nauk Medycznych 1, s. 5-9
*Krzysztof Krawczyński
Zakażenie wirusem zapalenia wątroby typu E: bieżące problemy i nowe kierunki badawcze
Hepatitis E: unresolved issues and research challenges
Distinguished Consultant, Division of Viral Hepatitis, Centers for Disease Control and Prevention Atlanta, GA, USA
Streszczenie
Odkrycie wirusa hepatitis E (HEV) w latach osiemdziesątych XX wieku zdefiniowało etiologicznie ostrą chorobę wirusową wątroby występującą w formie epidemii w środkowej i południowo wschodniej Azji, w Indiach, w Afryce i Chinach. W ostatnich latach, rzadkie przypadki zakażenia HEV obserwuje się w krajach Europy (Anglia, Francja, Holandia Niemcy) i bardzo sporadycznie w USA, w formie ostrej choroby zakaźnej z żółtaczką lub w postaci przewlekającego się zakażenia HEV. Bieżące i przyszłe badania nad epidemiologią i patogenezą zakażenia HEV powinny koncentrować się na określeniu przyczyn śmiertelności w grupie zakażonych kobiet w ciąży, na znalezieniu rezerwuaru wirusa w okresach między epidemiami, na określeniu znaczenia epidemiologicznego wirusa HEV zakażającego świnie i jego znaczenia w patologii wątroby u pacjentów z niepełnosprawnym układem immunologicznym (pacjenci zakażeni HEV po przeszczepach wątroby i nerek). Wysoki procent występowania przeciwciał anty-HEV w populacji krajów, w których poziom higieny osobistej i ogólnospołecznej wydaje się wykluczać możliwość rozprzestrzeniania się zakażenia HEV przez zainfekowane źródła wody jest problemem wymagającym nowych badań epidemiologicznych. Ostatnie prace laboratoryjne i kliniczne przybliżyły możliwość zapobiegania zapalenia wątroby typu E. Szczepionka, którą zastosowano w dużych grupach osób narażonych na zakażenie okazała się wysoce skuteczna. Słowa kluczowe: wirus zapalenia wątroby E, zapalenie wątroby, epidemiologia, świński HEV, szczepienia
Summary
This review presents the history of discovery of hepatitis E virus (HEV), global epidemiology, clinical course of hepatitis E in humans and results of experimental studies. Identification in 1983 HEV defined several epidemics of acute viral hepatitis of unknown etiology occurred in southeast and central Asia, India and Africa. Recently, sporadic cases of hepatitis E were confirmed in Europe and also in USA. Epidemiology and pathogenesis of hepatitis E is not very well known. The article presents results of clinical and basic studies on HEV and discuss the most interesting and still unsolved issues as: a) high mortality from HEV among pregnant women, b) the role of swine HEV in human, c) HEV source in immunocompromised individuals, and d) the casual relationship between HEV infection and liver pathology. Further study should be directed also to explain a relatively high frequency of anti-HEV antibody among healthy people in developed countries. Finally, the efficacy and perspectives of newly developed HEV vaccine are presented. Key words: hepatitis E virus, hepatitis, epidemiology, swine HEV, vaccination
Acute hepatitis E is a form of icteric, self-limited viral hepatitis caused by the hepatitis E virus (HEV). The disease prevails in developing countries of southeast Asia, Indian subcontinent and Africa. Rather surprisingly, a number of solitary cases or small series of cases of acute hepatitis E were recently identified in various European countries, the U.S. and Japan, where epidemic outbreaks of hepatitis E have never been reported and single cases of hepatitis E were confined to travelers returning from virus-endemic regions.
The disease, virus, epidemiology, and pathogenesis
Clinical manifestations of hepatitis E are similar to those observed in patients with acute hepatitis A or B (icteric hepatitis); the illness usually is insidious in onset with a prodromal phase lasting 1 to 4 days. Some of HEV-infected persons may exhibit only nonspecific symptoms resembling those of an acute viral febrile illness with serum alanine aminotransferase elevations but without jaundice (anicteric hepatitis), and some may remain entirely asymptomatic. A few patients may have a prolonged course with marked cholestasis (cholestatic hepatitis) lasting 2 to 6 months, ultimately with spontaneous resolution. In a small proportion of patients, the disease is severe and associated with subacute or fulminant hepatic failure. Population surveys during outbreaks have reported mortality rates of 0.07% to 0.6%.
Hepatitis E virus (HEV) was identified in 1983 by immune electron microscopy (1) and is classified in a separate genus named Hepevirus in family Hepeviridae (2). HEV is a small RNA virus, 32 to 34 nm in diameter, nonenveloped, and icosahedral. HEV RNA, cloned in 1990 and fully sequenced shortly thereafter, is approximately 7.2 kilobases in length, single- and positive-stranded, 5'-capped, and polyadenylated (3, 4). HEV genome contains three open reading frames (ORFs): ORF1 encodes nonstructural proteins, ORF2 encodes the viral capsid protein, and ORF3 encodes a protein of a little unknown function but essential for infectivity when tested in experimentally inoculated primates. Phylogenetic analysis of HEV isolates shows the existence of geographically distinct genotypes (see table adapted from (5)).
HEV infection is transmitted predominantly through the fecal-oral route; most reported outbreaks in disease endemic areas have been related to consumption of fecally contaminated drinking water. Several epidemics of hepatitis E affecting several hundred to several thousand persons have occurred on the Indian subcontinent and in southeast and central Asia, where this infection is endemic (6, 7). Outbreaks of hepatitis E have also been reported from the northern and western parts of Africa and the Middle East and the North America, where two small outbreaks in Mexico occurred in 1986 and 1987 (tab. 1). Overall attack rates range from 1% to 15% and are higher for adults (3% to 30%) than for children (0.2% to 10%). The male-to-female ratio among cases has ranged from 1:1 to 4:1 with a particularly high attack and mortality rates (up to 25%) among pregnant women. Recurrent epidemics in endemic regions are probably related to continuous fecal contamination of water because person-to-person transmission of hepatitis E seems to be uncommon, and secondary attack rates among household contacts are only 0.7% to 2.2%. Seroprevalence rates of anti-HEV are quite high among populations in endemic areas (10% to 40%), anti-HEV antibodies are uncommon in children and their rates increase during young adulthood to reach about 40% in adults (8). In contrast, antibodies are common in children in Egypt and seroprevalence rate reaches 70% or more in young adults (9) but epidemic outbreaks of hepatitis E are not known.
Table 1. Geographic distribution and infectivity of HEV genotypes.
Virologic, serological and pathologic data significant for pathogenesis of the HEV infection derived from both patients and experimentally infected primates (cynomolgus macaques, rhesus monkeys, and chimpanzees). In human volunteers (1, 10), the incubation period after oral exposure was 4 to 5 weeks and HEV was detected in the stool approximately 1 week before the onset of illness and for up to 2 weeks thereafter. In clinical cases, the fecal shedding of the virus was observed until about 4 weeks after the onset of illness.
In serum, HEV RNA can be detected for 2 weeks after the onset of illness in virtually all patients. The diagnosis of human HEV infection is based on detection of either HEV RNA in stool and serum specimens using a reverse transcription–polymerase chain reaction assay or the virus-specific host immune response (humoral and cellular). IgM anti-HEV appears in the early phase of clinical illness, lasts 4 to 5 months, and can be detected in 80% to 100% of cases during outbreaks of acute hepatitis E. The titer of IgG anti-HEV increases during the convalescent phase and remains high for at least 1 to 4.5 years; the exact duration of persistence of IgG anti-HEV in serum is not known.
The presence IgM anti-HEV in serum of patients is helpful in diagnosis of acute infection, whereas detection of IgG anti-HEV alone may indicate the convalescent phase or past infection (fig. 1). Patients with acute hepatitis E show cellular immune responses to HEV proteins (11, 12) but the number of cytokine producing HEV-reactive CD8-cells is not increased among peripheral blood mononuclear cells (PBMCs). Th1/Th2 cytokine balance is biased towards Th2 in pregnant women (13), in whom hepatitis E is particularly severe. The onset of ALT elevation in the serum and of histopathologic changes in the liver generally corresponds to the appearance of anti-HEV in serum. The temporal concordance of HEV-specific immune response with the onset of liver pathology suggests that liver injury is immune-mediated; there is no evidence for cytopathic properties of HEV itself. Histopathologic features of hepatitis E are similar to those of other forms of acute hepatitis but approximately one half of patients with hepatitis E has morphlogic evidence of cholestatic hepatitis (14). Submassive or massive necrosis and collapse of liver parenchyma are found in patients with severe liver injury.
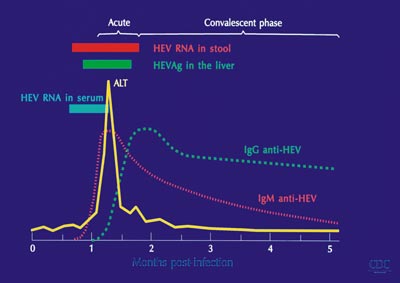 Fig. 1. Hepatitis E – virology, serology, and disease.
Current and future challenges to hepatitis E and HEV infection research
Mortality among HEV-infected pregnant women
Fulminant hepatic failure in HEV-infected pregnant women, associated with an increased frequency of abortions, stillbirths, and neonatal deaths, is an explosive disease with short pre-encephalopathy period, rapid development of cerebral edema and high occurrence of disseminated intravascular coagulation. Pregnant women, particularly those in the second or third trimester, are more frequently affected during hepatitis E outbreaks than are others in the population and have a worse outcome, with mortality rates of 5% to 25% (15, 16). The pathogenetic elements leading to severe liver damage during pregnancy, especially during its last trimester remains unknown and require further studies. Severe liver injury during pregnancy complicated by HEV infection may be related to immune and hormonal factors changed during pregnancy, and also to genetic and environmental factors in developing countries.
HEV reservoir in endemic regions
Recurrent epidemics of hepatitis E in disease-endemic regions almost always seem to be related to fecal contamination of water. HEV RNA was identified in waste water, sewage, and drinking water in endemic regions (17) and in occasional sewage specimens in nonendemic regions (18). It is not clear, however, where does the virus come from and for how long HEV remains viable in water sources. In the disease-endemic geographic regions, the presence of viable HEV in water sources may depends on the occurrence of continuous subclinical infections or prolonged fecal shedding of the virus by humans. Excretion of infectious HEV during the subclinical infection (without enzymatic evidence of liver pathology) has been demonstrated in the experimental macaque model of subclinical HEV infection (19). Reliable virology data on subclinical HEV infection in humans are scant and more surveillance studies are needed with application of newer technologies of HEV RNA detection (reverse transcriptase polymerase chain reaction – RT PCR) and quantification (real-time RT PCR).
Animal reservoirs and significance of zoonotic transmission of HEV
Epidemiologic significance of animal reservoir of HEV and zoonotic transmission of HEV to humans has been suggested since swine HEV, classified within genotype 3 of the four HEV genotypes, was identified in pigs in the Midwestern United States (20). Later on, swine HEV was found in pigs in several parts of the world and genomic sequences of HEV genotype 3 has also been isolated from other animals (wild boars, dear). The swine HEV-induced infection in pigs causes transient viremia, anti-HEV response and only insignificant liver pathology. Incidents of proven animal-to-human transmission of HEV genotype 3 have been shown to occur in Japan, where rare cases of hepatitis E developed after eating uncooked deer meat (21). In industrialized countries, transmission of HEV-like virus from pigs to humans seemed to be feasible because seroprevalence of anti-HEV among swine veterinarians and swine farmers was found to be significantly higher than in matched control populations. HEV contamination of the humans' food chain seems possible since HEV genetically related to the virus found in human cases of hepatitis E was isolated from pig livers sold in grocery stores in Japan and in the U.S. (22, 23). At the same time, however, clinically overt human HEV infections are rare in non-endemic areas despite the high prevalence of anti-HEV antibody among animals (domestic swine, wild rats and mice, cattle, dogs) and the prevalence of subclinical HEV infections in developed countries remains unknown. In summary, substantial evidence has been accumulated indicating that humans are susceptible to swine HEV similarly to primates infected experimentally. However, it would be highly questionable to consider acute hepatitis E in HEV-endemic geographic regions as a zoonotic disease. Genetic differences between human and animal HEV isolates were documented in India (24) and epidemic strains of HEV (genotype 1 and genotype 2) failed to induce infection in pigs (reverse cross-species challenge) when they were used for the experimental infections.
"Autochthonous” acute hepatitis E and seroprevalence of anti-HEV in developed countries
In nonendemic regions, hepatitis E is related mostly to travel to HEV-endemic regions and accounts for less than 1% of acute viral hepatitis. In recent years, however, solitary cases or small series of cases with indigenously-acquired cases of acute hepatitis E have been reported from United Kingdom, France, Netherlands, Germany and from the U.S. (25-28). HEV infection, caused by HEV genotype 3, with clinical characteristics of acute hepatitis in most cases, was thought to be locally-acquired and the patients were described as a group of "autochthonous” hepatitis E (25). Most patients had icterus, a few had anicteric illness with non-specific symptoms and some others were asymptomatic with transaminase elevations. The disease showed seasonal variations with peaks in spring and summer and appeared to be more common in residents of coastal and estuarine areas; hepatitis E was associated with shellfish consumption during an unusual outbreak among passengers of a cruise ship (29). For most "autochthonous” hepatitis E cases, however, HEV derived form pigs has been suggested as an infectious virus, although epidemiology of the virus transmission has not been determined. Equally unexplained is reason for recently reported high prevalence of anti-HEV among healthy people in the developed countries, in the USA around 20% (30) and 16% in United Kingdom (25). It remains unclear whether the presence of anti-HEV in non-endemic areas reflects subclinical HEV infection, serologic cross-reactivity with other agents, exposure to animal reservoirs of HEV-like viruses, or false-positive serologic results.
Chronic HEV infection and liver disease
Several epidemiologic and clinical observations from HEV endemic regions clearly indicate that hepatitis E virus (HEV) infection does not progress to chronic hepatitis. Recently, however, chronic HEV genotype 3 viremia has been reported in immunocompromised patients, such as renal and liver transplant recipients or patients undergoing chemotherapy. In a group of liver or renal transplant patients, HEV viremia and liver enzyme elevation appeared nearly at the same time, and liver biopsies showed evidence of liver inflammation with characteristics of chronic hepatitis (31). A case report published simultaneously suggested that such infection was concordant with the development of chronic hepatitis and cirrhosis (32). Furthermore, HEV genotype 3 infection has been proven in a few patients who had pathological changes in the liver biopsy specimens compatible with chronic hepatitis (interface hepatitis without fibrosis) in a large series of liver transplant patients tested for HEV infection in The Netherlands and Germany (33, 34). Further studies are needed to determine the source of the HEV infection in immunocompromised individuals and, most importantly, to establish a causal relationship between HEV infection and liver pathology.
Prevention of hepatitis E: perspectives for HEV vaccine
Cloning of the HEV genome and subsequent availability of recombinant proteins have led to the development of candidate HEV vaccines. Recently, an HEV vaccine (recombinant truncated form of HEV capsid protein) has undergone a phase 2, double-blind, randomized, placebo-controlled safety and efficacy trial among human volunteers in Nepal (35). Participants of the trial received three doses of either recombinant HEV protein or a matched placebo (at 0.1 and 6 months). The study subjects, nearly 2000 young mostly male (>99%) adults, underwent active surveillance for clinical acute hepatitis E for a period extending over 2 years.
The vaccine showed 95.5% protective efficacy against hepatitis E disease among those receiving 3 doses and 88.5% efficacy after administration of the first dose. The vaccine may be useful for pregnant women in the HEV endemic areas, for travelers to the HEV-endemic regions, and for persons with preexisting chronic liver disease who are known to be at a particular risk of liver disease worsening when infected with HEV. Cost considerations will determine whether this new and promising vaccine can be used in populations living in developing countries where hepatitis E is endemic and vaccination is likely to be the most beneficial. Piśmiennictwo
1. Balayan MS, Andjaparidze AG, Savinskaya SS et al.: Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983; 20: 23-31.
2. Berke T, Matson DO: Reclassification of the Caliciviridae into distinct genera and exclusion of hepatitis E virus from the family on the basis of comparative phylogenetic analysis. Arch Virol 2000; 145: 1421-36.
3. Reyes GR, Purdy MA, Kim JP et al.: Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 1990; 247: 1335-9.
4. Tsarev SA, Emerson SU, Reyes GR et al.: Characterization of a prototype strain of hepatitis E virus. Proc Natl Acad Sci USA 1992; 89: 559-63.
5. Schlauder GG, Frider B, Sookoian S et al.: Identification of 2 novel isolates of hepatitis E virus in Argentina. J Infect Dis 2000; 182: 294-7.
6. Vishwanathan R: Infectious hepatitis in Delhi (1955-56): A critical study: epidemiology. Indian J Med Res 1957; 45 (Suppl 1): 1-29.
7. Khuroo MS: Study of an epidemic of non-A, non-B hepatitis: possibility of another human hepatitis virus distinct from post-transfusion non- A, non-B type. Am J Med 1980; 68: 818-23.
8. Arankalle VA, Tsarev SA, Chadha MS et al.: Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis 1995; 171: 447-50.
9. Fix AD, Abdel-Hamid M, Purcell RH et al.: Prevalence of antibodies to hepatitis E in two rural Egyptian communities. Am J Trop Med Hyg 2000; 62: 519-23.
10. Chauhan A, Jameel S, Dilawari JB et al.: Hepatitis E virus transmission to a volunteer. Lancet 1993; 341: 149-50.
11. Naik S, Aggarwal R, Naik SR et al.: Evidence for activation of cellular immune responses in patients with acute hepatitis E. Indian J Gastroenterol 2002; 21: 149-52.
12. Shata MT, Barrett A, Shire NJ et al.: Characterization of hepatitis E-specific cell-mediated immune response using IFN-gamma ELISPOT assay. J Immunol Methods 2007; 328: 152-61.
13. Pal R, Aggarwal R, Naik SR et al.: Immunological alterations in pregnant women with acute hepatitis E. J Gastroenterol Hepatol 2005; 20: 1094-101.
14. Gupta DN, Smetana HF: The histopathology of viral hepatitis as seen in the Delhi epidemic (1955-56). Indian J Med Res 1957; 45 (Suppl): 101-13.
15. Khuroo MS, Teli MR, Skidmore S et al.: Incidence and severity of viral hepatitis in pregnancy. Am J Med 1981; 70: 252-5.
16. Bhatia V, Singhal A, Panda SK et al.: A 20-year single-center experience with acute lver failure during pregnancy: is the prognosis really worse? Hepatology 2008; 48: 1577-85.
17. Jothikumar N, Aparna K, Kamatchiammal S et al.: Detection of hepatitis E virus in raw and treated wastewater with the polymerase chain reaction. Appl Environ Microbiol 1993; 59: 2558-62.
18. Pina S, Jofre J, Emerson SU et al.: Characterization of a strain of infectious hepatitis E virus isolated from sewage in an area where hepatitis E is not endemic. Appl Environ Microbiol 1998; 64: 4485-8.
19. Aggarwal R, Kamili S, Spelbring J et al.: Experimental studies on subclinical hepatitis E virus infection in cynomolgus macaques. J Infect Dis 2001; 184: 1380-5.
20. Meng XJ, Purcell RH, Halbur PG et al.: A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA 1997; 94: 9860-5.
21. Tei S, Kitajima N, Takahashi K et al.: Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003; 362: 371-3.
22. Yazaki Y, Mizuo H, Takahashi M et al.: Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J Gen Virol 2003; 84: 2351-7.
23. Feagins AR, Opriessnig T, Guenette DK et al.: Detection and characterization of infectious Hepatitis E virus from commercial pig livers sold in local grocery stores in the USA. J Gen Virol 2007; 88: 912-7.
24. Shukla P, Chauhan UK, Naik S et al.: Hepatitis E virus infection among animals in northern India: an unlikely source of human disease. J Viral Hepat 2007; 14: 310-7.
25. Dalton HR, Stableforth W, Thurairajah P et al.: Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol 2008; 20: 784-90.
26. Peron JM, Mansuy JM, Poirson H et al.: Hepatitis E is an autochthonous disease in industrialized countries. Analysis of 23 patients in South-West France over a 13-month period and comparison with hepatitis A. Gastroenterol Clin Biol 2006; 30: 757-62.
27. Waar K, Herremans MM, Vennema H et al.: Hepatitis E is a cause of unexplained hepatitis in The Netherlands. J Clin Virol 2005; 33: 145-9.
28. Wichmann O, Schimanski S, Koch J et al.: Phylogenetic and case-control study on herpatitis E virus infection in Germany. J Infect Dis 2008; 198: 1732-41.
29. Said B, Ijaz S, Kafatos G et al.: Hepatitis E outbreak on cruise ship. Emerg Infect Dis 2009; 15: 1738-44.
30. Kuniholm MH, Purcell RH, McQuillan GM et al.: Epidemiology of hepatitis E virusd in the United States: Results from the Third National Health and Nutrition Examination Survey, 1988-1994 2009; 200:48-56.
31. Kamar N, Selves J, Mansuy JM et al.: Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008; 358: 811-7.
32. Gerolami R, Moal V, Colson P: Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N Engl J Med 2008; 358: 859-60.
33. Haagsma E, Niesters HGM, van der Berg AP et al.: Prevalence of hepatitis E virus infection in liver transplant recipients. Liver Transpl 2009; 15: 1225-28.
34. Pischke S, Suneetha PV, Baechlein C et al.: Hepatitis E virus infection as a cause of graft hepatitis in liver transplant recipients. Liver Transpl 2009; in press.
35. Shrestha MP, Scott RM, Joshi DM et al.: Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med 2007; 356: 895-903.
otrzymano/received: 2009-10-30 zaakceptowano/accepted: 2009-12-04 Adres/address: *Krzysztof Krawczyński Experimental Pathology Laboratory, Division of Viral Hepatitis, NCHHSTP Centers for Disease Control and Prevention 1600 Clifton Road, Bldg 18, 3-134 Atlanta GA 30333, USA e-mail: kzk1@cdc.gov Artykuł Zakażenie wirusem zapalenia wątroby typu E: bieżące problemy i nowe kierunki badawcze w Czytelni Medycznej Borgis. |
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








