|
© Borgis - Postępy Nauk Medycznych 9, s. 706-713
Tomasz Dębski, Lubomir Lembas, *Józef Jethon
Basal cell carcinoma. Current views (Part II). Diagnostics and treatment
Rak podstawnokomórkowy skóry. Współczesne poglądy (Część II). Diagnostyka, leczenie
Department of Plastic Surgery, The Medical Centre of Postgraduate Education in Warsaw
Head of the Department: Józef Jethon, MD, PhD, Professor of Plastic Surgery Streszczenie
The gold standard in BCC diagnostics is a biopsy combined with a histopathological examination, the result of which is crucial. Depending on a method of collecting diagnostic material, biopsies can be divided into: shave biopsy, punch biopsy, wedge biopsy, excisional biopsy and curettage. Apart from biopsies BCC diagnosis can also be supported by imaging techniques such as dermatoscopy allowing for distinguishing pigmented BCC from melanoma, computed tomography or magnetic resonance imaging which are used in order to determine the infiltration of BCC when it is suspected that lesion adjacent structures (cartilage, bone, large nerves, orbital area or parotid gland) can be affected. Other modern imaging techniques are only of academic interest and do not play important roles in clinical diagnostics. They include: ultrasound examination, optical coherence tomography (OCT), confocal microscopy (RCM, FLSM), photodynamic diagnostics (PDD), spectrometric diagnostics and fluorescence lifetime imaging microscopy (FLIM). BCC treatment is managed by physicians of different specialities (dermatologists, plastic surgeons, general surgeons, oncologists, ophthalmologists, ENT specialists, and even general medicine specialists) who promote therapeutic options which are closely related to their specialities and are often controversial. Available literature reports different therapeutic methods including non-invasive techniques such as local application of Imiquimod, photodynamic therapy, radiation therapy, CO2 laser ablation, cryosurgery, curettage and cautery or surgical excision with a margin of clinically normal surrounding tissue. Functional and aesthetic results, treatment efficacy, side effects and effects on the quality of life are different and depend on the method that has been used. In order to select an appropriate method American and British therapeutic guidelines presented in this article might be helpful. Słowa kluczowe: basal cell carcinoma, diagnostics, biopsy, therapeutic guidelines
Summary
Złotym standardem w diagnostyce BCC jest biopsja z następowym badaniem histopatologicznym, którego wynik decyduje o rozpoznaniu. Ze względu na sposób pobierania materiału diagnostycznego wyróżnia się biopsję ścinająca, sztancową, klinową, wycinającą oraz łyżeczkowanie. Poza biopsją w rozpoznawaniu BCC pomocne są również techniki obrazowe takie jak: dermatoskopia, która pozwala na odróżnienie formy barwnikowej BCC od czerniaka, tomografia komputerowa lub rezonans magnetyczny, które stosuje się w celu określenia zakresu nacieku nowotworowego przy podejrzeniu zajęcia struktur graniczących ze zmianą (chrząstka, kość, duże nerwy, oczodół lub ślinianka przyuszna). Pozostałe nowoczesne metody diagnostyki obrazowej są w kręgu zainteresowań akademickich i nie odgrywają znaczącej roli w diagnostyce klinicznej. Należą do nich: USG, tomografia optyczna z użyciem światła częściowo spójnego (OCT), technika mikroskopii konfokalnej (RCM, FLSM), diagnostyka fotodynamiczna (PDD), diagnostyka spektrometryczna oraz metody obrazujące czas trwania fluorescencji (FLIM). Leczeniem BCC zajmują się lekarze wielu specjalności (dermatolodzy, chirurdzy plastycy, chirurdzy ogólni, onkolodzy, okuliści, laryngolodzy, a nawet interniści) promując najbliższe swojej specjalności i często kontrowersyjne algorytmy leczenia. W dostępnej literaturze przedstawiane są różne metody leczenia począwszy od nieinwazyjnych takich jak miejscowe stosowanie maści zawierającej Imiquimod, terapii fotodynamicznej, radioterapii poprzez ablację laserem CO2, kriochirurgię, elektrokoagulację a skończywszy na łyżeczkowaniu czy chirurgicznym wycięciu zmiany z marginesem makroskopowo zdrowych tkanek. Wynik funkcjonalny i estetyczny, skuteczność leczenia, działania uboczne oraz wpływ na jakość życia pacjenta są różne i zależą od zastosowanej metody. W dokonaniu wyboru właściwej metody pomocne będą przedstawione w niniejszym artykule amerykańskie i brytyjskie wytyczne leczenia BCC. Key words: rak podstawnokomórkowy skóry, diagnostyka, biopsja, wytyczne leczenia
BCC diagnostics
Invasive diagnostics (biopsy)
Despite the fact that clinical features are well described and relatively specific, it should be emphasised that only a result of a histopathological examination can confirm the diagnosis and a biopsy is the gold standard in BCC diagnostics. Some specialists recommend to perform a biopsy in all cases when BCC is suspected. Others recommend it only in diagnostically doubtful cases or when a histological type can affect the choice of a therapeutic method and prognosis (1). There are following types of a biopsy:
Curettage – involves removing neoplastic tissue using a special spoon. Due to the fact that the internal structure of curetted tissues is lost this method is not reliable and currently not recommended (2).
Shave biopsy – involves cutting the half-thickness skin at a tangent to the skin. It is recommended for superficial BCC, especially if a tumour is multifocal, is present in regression areas and in the case of recurrent disease. A shave biopsy allows for collecting material from a large area. The wound heals fast and leaves no secondary deformations, therefore it can be difficult to locate when a patient returns to continue treatment (a biopsy site should be marked and photographed) (2).
Punch biopsy (trepanobiopsy) – a full-thickness skin fragment with the diameter of 4-5 mm is removed with a punch consisting of a metal tube with a sharp edge and a handle.
It is recommended in diagnostics of lesions located in aesthetically and functionally important skin regions (e.g. face.) The repetitive 2-mm punch biopsy may be used to determine poorly demarcated neoplasms (2).
Incisional biopsy – involves removing a lesion fragment with a healthy skin fragment of usually about 3-4 mm. It is recommended in highly advanced cases and in recurrent disease. It allows for the estimation of infiltration depth, which is of special importance before radiation therapy (2).
Excisional biopsy – is a diagnostic and therapeutic method. It is recommended in all cases where a defect formed after lesion excision can be sutured without leaving deformities (trunk, limbs). Not only does it provide information regarding final diagnosis but also informs about the treatment efficacy (completeness). It involves primary excision of a lesion with a margin of clinically normal surrounding tissues. The recommended margin ranges from 2 to 8 mm. Unfortunately, it occurs quite often that clinically normal surrounding tissues are saved to too large an extent and excision is not complete, and as a result neoplastic recurrence is observed (2).
Imaging diagnostics
Dermatoscopy includes observing the skin with a dermatoscope consisting of a microscope with 10-100x magnification. In addition, a dermatoscope is equipped with an internal light source that illuminates the skin at an angle of 20°, therefore the picture is enlarged and its resolution is higher. This method is of the greatest importance in the diagnostics of pigmented lesions and melanoma. It makes it possible to distinguish melanoma from pigmented BCC. According to the Monzi criteria pigmented BCC has the following features: lack of pigment network and the presence of one of the following features: maple leaflike areas, spoke wheel areas, large gray-blue ovoid nests, large gray-blue globules, telangiectasias with arborisation and ulceration (3). In the case of nodular BCC in 82% it is possible to observe branching vessels, and the superficial form has delicate and short telangiectasias (4).
Imaging tests such as computed tomography or magnetic resonance imaging are used to determine the extent of neoplastic infiltration when cartilage, bone, large nerve (5), eyeball (6, 7) or parotid gland (8) involvement is suspected.
Other modern methods are only of academic interest and do not play important roles in clinical diagnostics. They include:
High resolution ultrasound examination (20-100 Hz) allowing for imaging the skin up to the depth of even 1.1 mm which may be helpful during evaluation of the depth of lesion infiltration (9).
Optical coherence tomography (OCT) (10) – the mechanism of action is similar to the ultrasound examination; however, infrared light is used instead of ultrasounds – it is possible to visualise skin layers, appendages and vessels (11); nevertheless, it is not possible to see the basement membrane or to evaluate the depth of infiltration (12).
Confocal microscopy (RCM, reflectance confocal microscopy and FLSM, fluorescence confocal laser scanning microscopy) (3) – involving the detection of endogenous (RCM) or exogenous (FLSM) dye with special light, what makes it possible to visualise cells and cell structures with the precision nearly as good as the one of a histopathological examination (14, 15).
Photodynamic diagnostics (PDD) involves the detection of light (using electromagnetic waves) emitted from tissues after fluorescence excitation (16).
Spectrometric diagnostics involves differentiation and evaluation of the excitation spectrum of healthy tissue and tissues with dysplastic or neoplastic lesions (17).
Fluorescence lifetime imaging methods (FLIM) – this time is different for healthy tissues and neoplastic tissues (18).
BCC: therapeutic options
BCC treatment is managed by physicians of many specialities. Dermatologists often use cryotherapy and remove small lesions located on the face and trunk, small eyelid neoplasms belong to ophthalmologists, lesions on the nose and ear are resected by ENT specialists, whereas dental surgeons remove lesions located in the area of the mouth cavity. Lesions the size of which makes it possible to direct closure are also excised by general surgeons and even by general practitioners in some countries (2).
In the treatment of more extensive and advanced BCC cases in order to cover a defect after neoplasm excision it is necessary to use different reconstruction methods, what is possible only in specialist centres of plastic, maxillary or oncological surgery.
As there are many specialists managing BCC treatment, there are also numerous and different therapeutic options. They can be divided into destructive methods where neoplastic tissue is destroyed and it is not possible to evaluate a histopathological type or procedure completeness, and surgical methods involving the excision of neoplastic tissue with a margin of clinically normal surrounding tissues.
Destructive methods include:
? local immunotherapy using imiquimod-containing ointment that stimulates and intensifies a local anti-inflammatory reaction what leads to BCC regression (19).
? photodynamic therapy, which uses the fact that some photosensitive substances, namely substances absorbing light of specific wavelength accumulate in neoplastic tissues. When they are selectively accumulated in the neoplastic tissue the lesion is exposed to laser light at the wavelength absorbed by a given photosensitive substance. Consequently, the high levels of radiation energy are accumulated in the neoplastic tissue and it becomes destroyed (16).
? radiation therapy, combining many methods starting from superficial radiation (up to 170 kV) in the case of lesions with the depth up to 6 mm, to electron beam radiation and brachytherapy (20).
? curettage, which is often combined with other methods such as: coagulation (21), imiquimod (22), photodynamic therapy (23), cryosurgery (24) or surgery (25, 26).
? cryosurgery, which involves the destruction of neoplastic tissue during one or several cycles of freezing using liquid nitrogen (27).
? CO 2 laser is a new therapeutic option still studied in clinical trials (20).
? local application of fluorouracil (5-FU)-containing ointment, which is a classic cytostatic. Further studies are necessary to evaluate its long-term efficacy (20).
The destructive methods presented above may be used only in some highly selected cases. Appropriate patient qualification combined with experience of a specialist using a given therapeutic method may provide good therapeutic effects.
As BCC, contrary to other malignant neoplasms, is rarely responsible for patient´s death, 5-year survival as an outcome measure is not justified. For that reason, in order to assess the efficacy of BCC treatment the recurrence rate during a 5-year follow-up period is used. Moreover, it is necessary to take cosmetic and functional results and treatment comfort into account.
Currently it is difficult to evaluate unanimously which destructive method is the best.
It is a result of the fact that current literature lacks in prospective trials comparing different methods and that the criteria of patients´ qualification are extremely narrow and suitable only for individual methods, therefore it is not possible to compare them objectively. It is commonly thought that each method is good if it is applied in an appropriate case by an experienced specialist.
Surgical methods include classic excision of a lesion with a margin of clinically normal surrounding tissues and Mohs micrographic surgery that involves staged resection of a lesion with intraoperational histopathological evaluation of its edges (Part III).
Selecting a therapeutic method
In order to determine the indications for different methods, prognostic factors predicting BCC with a high-risk of recurrence have been identified.
Prognostic factors
Tumour site – a significantly higher risk of recurrence compared to the trunk and neck (recurrence in 0.5%) was observed for BCC located on the face (lateral canthal region – 43% of recurrence, upper lid and eyebrow 33%, nose 19%), ear (24%) and scalp. Some specialists think that it is associated with a specifical anatomical structure of the subcutaneous tissue of these areas, which creates a risk of deeper invasion (28).
Other specialists make attempts to explain a higher risk of recurrence in the central face (17.5% of recurrence vs 8.6% for other parts of the face) by the fact that a neoplasm changes its way of spreading from horizontal to vertical, what is reflected by the fact that infiltration spreads along embryonic connections between facial buds (29, 30).
Based on the risk of recurrence, the following areas can be distinguished: high risk of recurrence (H), middle risk of recurrence (M) and low risk (L) (Fig. 1).
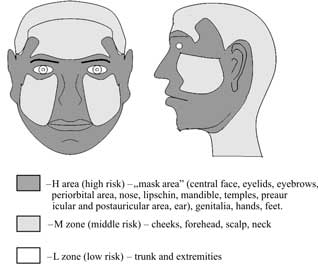 Fig. 1. Recurrence risk areas (31).
However, it has to be emphasised that the above division into risk areas is based on a series of retrospective studies that analysed recurrence sites following surgical treatment. The margin of a clinically normal surrounding tissues resected with a lesion was not taken into account.
According to the authors more frequent recurrences in the H zone may be associated with the fact that a smaller margin of clinically normal surrounding tissues is applied in these face areas. It is a result of the fact that operators are afraid of poor aesthetical and functional effects. For example, applying a larger margin in the medial canthal region could deform and disturb the functions of the eye protective apparatus. In other risk areas (M or L area) e.g. on the neck or the trunk it is possible to resect lesions with larger margins of clinically normal surrounding tissues, what has no significant effects on aesthetical and functional results; however, it will significantly increase completeness of excision.
Tumour size and depth of invasion – the recurrence rate increases with the increasing size of a tumour (tab. 1) Depending on a diameter the recurrence rate in a 5-year follow-up period is as follows for the following tumour sizes: <1.5 cm-12%,>3 cm-23.1% (33). In other studies: <1 cm-3.2%, 1-2 cm-8%,>2cm recurrence in almost 1/3 of cases (29, 30)
Table 1. The recurrence rate in a 5-year follow-up period depending on a tumour size (32).
Moreover, invasion of structures lying under the skin such as cartilages and bones is associated with a higher risk of recurrence.
The guidelines of National Comprehensive Cancer Network (NCCN), an American organisation studying therapeutic algorithms for treatment of neoplasms, combine tumour site and size in order to assess the recurrence risk (diagram 1).
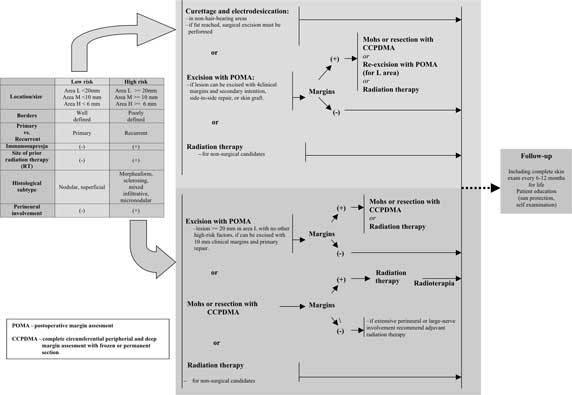 Diagram 1. Guidelines for BCC treatment based on NCNN Clinical Practice Guidelines in Oncology: Basal Cell and Squamous Cell Skin Cancers (USA, 01.2009).
Definition of clinical margins – the recurrence risk for lesions with ill-defined clinical tumor borders is higher than for lesions with well-defined borders (35).
Histological subtype of BCC – for some morphological subtypes, e.g. morpheaform, infiltrating, micronodular or mixed types, the recurrence risk is higher (tab. 2) (29, 36).
Table 2. Clinical-pathological classification of BCC (WHO 2006) and classification based on the recurrence risk (37, 38).
Lesion excision with a classic margin is incomplete in 6.4% for a nodular lesion, for a superficial lesion – 3.6%, for a micronodular lesion – 18.6%, for an infiltrating lesion – 26%, for a morpheaform lesion – 33.3% (28). Moreover, histological features of infiltration, especially perineural and perivascular involvement, are significant risk factors of recurrence (36).
Recurrent BCC – treatment of recurrent BCC is associated with a significantly higher recurrence rate than treatment of primary lesions (Part III.) (2).
Immunosuppression – is associated not only with a higher risk of BCC in general, but also with a higher recurrence rate (39).
BCC in patients after organ transplantation and leukaemic patients constitute a special problem (Part I).
Other prognostic factors, which are mentioned more rarely: age <35 years (40), a reconstruction method after surgical excision (recurrent disease within the first year when full-thickness skin is grafted, after two years when split-thickness skin is grafted and within 4 years after local tissue transfers) (41, 42). Prognosis for Gorlin syndrome treatment is also poor (43).
The presence or lack of such features makes it possible to divide all types of BCC into high or low-risk lesions, therefore it is possible to select an appropriate therapeutic method. The latest guidelines for BCC treatment suggested by the British and American Scientific Societies are outlined (diagram 1 and 2).
 Diagram 2. Treatment of BCC based on Guidelines for the Management of Basal Cell Carcinoma (UK, 07.2008 r.).
As it can be concluded from the guidelines, all high- and low-risk BCC as well as recurrent BCC may be surgically treated.
Surgery is currently the gold standard for BCC treatment because of high efficacy, its versatility, fast results, good aesthetical and functional outcome, low risk of complication, availability (the majority of mentioned destructive methods can only be applied in highly specialized clinics), low costs of treatment and what is even more important, the possibility to evaluate procedure completeness, (Part III) (44).
Piśmiennictwo
1. Costantino D, Lowe L, Brown DL: Basosquamous carcinoma – an under-recognized, high-risk cutaneous neoplasm: case study and review of the literature. J Plast Reconstr Aesthet Surg 2006; 59: 424-8.
2. Australian Cancer Network Management of Non-Melanoma Skin Cancer Working Party. Non-melanoma skin cancer: Guidelines for treatment and management in Australia; 24 October 2002.
3. Menzies SW et al.: Surface microscopy of pigmented basal cell carcinoma. Arch. Dermatol. 2000; 136(8): 1012-1016.
4. Argenziano G et al.: Vascular structures in skin tumors: a dermoscopy study. Arch Dermatol 2004; 140(12): 1485-1489.
5. Williams LS, Mancuso AA, Mendenhall WM: Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys 2001; 49: 1061-9.
6. Leibovitch I et al.: Orbital invasion by periocular basal cell carcinoma. Ophthalmology 2005; 112: 717-23.
7. Meads SB, Greenway HT: Basal cell carcinoma associated with orbital invasion: clinical features and treatment options. Dermatol Surg 2006; 32: 442-6.
8. Farley RL, Manolidis S, Ratner D: Aggressive basal cell carcinoma with invasion of the parotid gland, facial nerve, and temporal bone. Dermatol Surg 2006; 32: 307-15.
9. Vogt M, Ermert H: High Resolution Ultrasound In: Bioengineering of the Skin. Skin, Skin imaging and Analysis; second edition, KP Wilhelm, P Elsner, E Berardesca, H Maibach (eds.). Informa Healthcare USA Inc, NY, USA, 2007; 17-29.
10. Olmedo JM et al.: Optical coherence tomography for the characterization of basal cell carcinoma in vivo: a pilot study. J Am Acad Dermatol 2006; 3: 408-412
11. Welzel J et al.: Optical coherence tomography of the human skin. J Am Acad Dermatol 1997; 37: 958-63.
12. Welzel J, Bruhns m, Wolff: Optical coherence tomography in contact dermatitis and psoriasis. Arch Dermatol Res 2003; 295: 50-5.
13. Ulrich M et al.: Noninvasive Diagnosis of Non-melanoma Skin Cancer: Focus on Reflectance Confocal Microscopy, Expert Rev Dermatol 2008; 3(5): 557-567.
14. Swindle LD et al.: View of normal human skin in vivo as observed using fluorescent fiber-optic confocal microscopic imaging. J Invest Dermatol 2003a; 121: 706-12.
15. Swindle L et al.: Fluorescence confocal microscopy of normal human skin and skin lesions in vivo. Skin Res Technol 2003b; 9: 167.
16. Morawiec Z i wsp.: Czy metoda fotodynamiczna będzie diagnostyką przyszłości? Acta Clinica et Morphologica 2004; 7(1): 10-14.
17. Sieroń A, Gibiński P, Woźnica T: Spektrometryczny system wczesnego wykrywania i diagnostyki nowotworów. Acta Bio-Optica et Informatica Medica 2007; 13(4).
18. Galletly NP et al.: Fluorescence Lifetime Imaging Distinguishes Basal Cell Carcinoma From Surrounding Uninvolved Skin; Br J Dermatol 2008; 159(1): 152-161.
19. Marks R et al.: Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: results of a multicenter 6-week dose–response trial. J Am Acad Dermatol 2001; 44: 807-13.
20. Telfer NR, Colver GB, Morton CA: Guidelines for the Management of Basal Cell Carcinoma. Br J Dermatol 2008; 158(7): 35-48.
21. Reymann F: 15 years´ experience with treatment of basal cell carcinomas of the skin with curettage. Acta Derm Venereol (Stockh) 1985; 120 (Suppl.): 56-9.
22. Wu JK, Oh C, Strutton G, Siller G: An open-label, pilot study examining the efficacy of curettage followed by imiquimod 5% cream for the treatment of primary nodular basal cell carcinoma. Australas J Dermatol 2006; 47: 46-8.
23. Soler AM et al.: A follow-up study of recurrence and cosmesis in completely responding superficial and nodular basal cell carcinomas treated with methyl 5-aminolaevulinate-based photodynamic therapy alone and with prior curettage. Br J Dermatol 2001; 145: 467-71.
24. Nordin P, Stenquist B: Five-year results of curettage-cryosurgery for 100 consecutive auricular non-melanoma skin cancers. J Laryngol Otol 2002; 116: 893-8.
25. Johnson TM, Tromovitch TA, Swanson NA: Combined curettage and excision: a treatment method for primary basal cell carcinoma. J Am Acad Dermatol 1991; 24: 613-17.
26. Chiller K et al.: Efficacy of curettage before excision in clearing surgical margins of nonmelanoma skin cancer. Arch Dermatol 2000; 136: 1327-32.
27. Graham G: Statistical data on malignant tumors in cryosurgery: 1982. J Dermatol Surg Oncol 1983; 9: 238-9.
28. Mooney M, Parry E: Mohs Micrographic Surgery 2007: www.emedicine.com/derm/topic542.htm
29. Włodarkiwicz A, Muraszko-Kuźma M: Czynniki zwiększonego ryzyka wznowy w raku podstawnokomórkowym skóry. Przegl Dermatol 1998; 85, 405.
30. Wronkowski Z, Dąbska M, Kułakowski A: Rak skóry. PZWL, Warszawa 1978.
31. Swanson NA: Mohs surgery: Technique, indications, applications, and the future. Arch. Dermatol 1983; 119: 761-773.
32. Silverman MK et al.. Recurrence rates of treated basal cell carcinomas. Part 3: Surgical excision. J Dermatol Surg Oncol 1992; 18: 471-476.
33. Dubin N, Kopf AW: Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol 1983; 119 (5): 373-7.
34. NCNN Clinical Practice Guidelines in Oncology: Basal Cell and Squamous Cell Skin Cancers V.I.2009: www.nccn.org
35. Rowe DE, Carroll RJ, Day CL: Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol 1989; 15; 315-328.
36. Costantino D, Lowe L, Brown DL: Basosquamous carcinoma – an under-recognized, high-risk cutaneous neoplasm: case study and review of the literature. J Plast Reconstr Aesthet Surg 2006; 59: 424-8.
37. Kossard S et al.: Basal cell carcinoma. In: LeBoit P, Burg G, Weedon D, Sarasin A. The WHO classification of tumours. Pathology and genetics of skin tumours. IARC Press, Lyon 2006: 13-19.
38. Bieniek A, Cisło M, Jankowska-Konsur A. Nowotwory skóry. Klinika, patologia, leczenie, Galaktyka 2008.
39. Gupta AK, Cardella CJ, Haberman HF: Cutaneus malignant neoplasms In patients with renal transplants. Arch Dermat 1986; 122; 1288-1293.
40. Boeta-Angeles L, Bennet RG: Features associated with recurrence. Cutaneus Oncology. Malden: Blackwell Science; 1998: 998.
41. Richmond JD, Davie RM: The significance of incomplete excision in patients with basal cell carcinoma. Br J Plast Surg 1987; 40; 63-67.
42. Koplin L, Zarem HA: Recurrent basal cell carcinoma: a review concerning the incidence, behavior, and management of recurrent basal cell carcinoma, with emphasis on the incompletely excised lesion. Plast Reconstr Surg 1980; 65: 656-64.
43. Gorlin RJ: Nevoid basal cell carcinoma syndrome. Dermatol Clin 1995; 13: 113-125.
44. Bath-Hextall F etr al.: Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev 2007; 1: CD003412.
otrzymano/received: 2009-05-21 zaakceptowano/accepted: 2009-08-12 Adres/address: *Józef Jethon Department of Plastic Surgery, The Medical Centre of Postgraduate Education in Warsaw Czerniakowska 231 Str., 00-416 Warsaw tel.: (0-22) 584-11-91 e-mail: jjethon@szpital-orlowskiego.pl The whole paper Basal cell carcinoma. Current views (Part II). Diagnostics and treatment is also available at On-line Medical Library. |
Chcesz być na bieżąco? Polub nas na Facebooku: strona Wydawnictwa na Facebooku |








